Echothiophate Iodide
Editor-In-Chief: C. Michael Gibson, M.S., M.D. [1]; Associate Editor(s)-in-Chief: Aparna Vuppala, M.B.B.S. [2]
Disclaimer
WikiDoc MAKES NO GUARANTEE OF VALIDITY. WikiDoc is not a professional health care provider, nor is it a suitable replacement for a licensed healthcare provider. WikiDoc is intended to be an educational tool, not a tool for any form of healthcare delivery. The educational content on WikiDoc drug pages is based upon the FDA package insert, National Library of Medicine content and practice guidelines / consensus statements. WikiDoc does not promote the administration of any medication or device that is not consistent with its labeling. Please read our full disclaimer here.
Overview
Echothiophate Iodide is an antiglaucoma that is FDA approved for the treatment of chronic open-angle glaucoma. Subacute or chronic angle-closure glaucoma after iridectomy or where surgery is refused or contraindicated. Common adverse reactions include frontal headache, headache, blurred vision, Burning sensation in eye, iritis, night blindness, uveitis.
Adult Indications and Dosage
FDA-Labeled Indications and Dosage (Adult)
Glaucoma
- Chronic open-angle glaucoma. Subacute or chronic angle-closure glaucoma after iridectomy or where surgery is refused or contraindicated. Certain non-uveitic secondary types of glaucoma, especially glaucoma following cataract surgery.
Accommodative Esotropia
- Concomitant esotropias with a significant accommodative component.
Dosing Information
Glaucoma
- Selection of Therapy – The medication prescribed should be that which will control the intraocular pressure around-the-clock with the least risk of side effects or adverse reactions. “Tonometric glaucoma” (ocular hypertension without other evidence of the disease) is frequently not treated with any medication, and echothiophate iodide for ophthalmic solution is certainly not recommended for this condition. In early chronic simple glaucoma with field loss or disc changes, pilocarpine is generally used for initial therapy and can be recommended so long as control is thereby maintained over the 24 hours of the day.
- When this is not the case, echothiophate iodide for ophthalmic solution 0.03% may be effective and probably has no greater potential for side effects. If this dosage is inadequate, epinephrine and a carbonic anhydrase inhibitor may be added to the regimen. When still more effective medication is required, the higher strengths of echothiophate iodide for ophthalmic solution may be prescribed with the recognition that the control of the intraocular pressure should have priority regardless of potential side effects. In secondary glaucoma following cataract surgery, the higher strengths of the drug are frequently needed and are ordinarily very well tolerated.
- The dosage regimen prescribed should call for the lowest concentration that will control the intraocular pressure around-the-clock. Where tonometry around-the-clock is not feasible, it is suggested that appointments for tension-taking be made at different times of the day so that inadequate control may be more readily detected. Two doses a day are preferred to one in order to maintain as smooth a diurnal tension curve as possible, although a single dose per day or every other day has been used with satisfactory results. Because of the long duration of action of the drug, it is never necessary or desirable to exceed a schedule of twice a day. The daily dose or one of the two daily doses should always be instilled just before retiring to avoid inconvenience due to the miosis.
- Early Chronic Simple Glaucoma – Echothiophate iodide for ophthalmic solution 0.03% instilled twice a day, just before retiring and in the morning, may be prescribed advantageously for cases of early chronic simple glaucoma that are not controlled around-the-clock with other less potent agents. Because of prolonged action, control during the night and early morning hours may then sometimes be obtained. A change in therapy is indicated if, at any time, the tension fails to remain at an acceptable level on this regimen.
- Advanced Chronic Simple Glaucoma and Glaucoma Secondary to Cataract Surgery – These cases may respond satisfactorily to echothiophate iodide for ophthalmic solution 0.03% twice a day as above. When the patient is being transferred to echothiophate iodide for ophthalmic solution because of unsatisfactory control with pilocarpine, carbachol, epinephrine, etc., one of the higher strengths, 0.06%, 0.125%, or 0.25% will usually be needed. In this case, a brief trial with the 0.03% eyedrops will be advantageous in that the higher strengths will then be more easily tolerated.
- Concomitant Therapy –
- Echothiophate iodide for ophthalmic solution may be used concomitantly with epinephrine, a carbonic anhydrase inhibitor, or both.
- Technique –
- Good technique in the administration of echothiophate iodide for ophthalmic solution requires that finger pressure at the inner canthus should be exerted for a minute or two following instillation of the eyedrops, to minimize drainage into the nose and throat. Excess solution around the eye should be removed with tissue and any medication on the hands should be rinsed off.
Accommodative Esotropia (Pediatric Use)
- In Diagnosis –
- One drop of 0.125% may be instilled once a day in both eyes on retiring, for a period of two or three weeks. If the esotropia is accommodative, a favorable response will usually be noted which may begin within a few hours.
- In Treatment –
- Echothiophate iodide for ophthalmic solution is prescribed at the lowest concentration and frequency which gives satisfactory results. After the initial period of treatment for diagnostic purposes, the schedule may be reduced to 0.125% every other day or 0.06% every day. These dosages can often be gradually lowered as treatment progresses. The 0.03% strength has proven to be effective in some cases. The maximum usually recommended dosage is 0.125% once a day, although more intensive therapy has been used for short periods.
- Technique –
- Duration of Treatment – In diagnosis, only a short period is required and little time will be lost in instituting other procedures if the esotropia proves to be unresponsive. In therapy, there is no definite limit so long as the drug is well tolerated. However, if the eyedrops, with or without eyeglasses, are gradually withdrawn after about a year or two and deviation recurs, surgery should be considered. As with other miotics, tolerance may occasionally develop after prolonged use. In such cases, a rest period will restore the original activity of the drug.
Off-Label Use and Dosage (Adult)
Guideline-Supported Use
There is limited information regarding Off-Label Guideline-Supported Use of Echothiophate Iodide in adult patients.
Non–Guideline-Supported Use
There is limited information regarding Off-Label Non–Guideline-Supported Use of Echothiophate Iodide in adult patients.
Pediatric Indications and Dosage
FDA-Labeled Indications and Dosage (Pediatric)
There is limited information regarding FDA-Labeled Use of Echothiophate Iodide in pediatric patients.
Off-Label Use and Dosage (Pediatric)
Guideline-Supported Use
There is limited information regarding Off-Label Guideline-Supported Use of Echothiophate Iodide in pediatric patients.
Non–Guideline-Supported Use
There is limited information regarding Off-Label Non–Guideline-Supported Use of Echothiophate Iodide in pediatric patients.
Contraindications
- Active uveal inflammation.
- Most cases of angle-closure glaucoma, due to the possibility of increasing angle block.
- Hypersensitivity to the active or inactive ingredients.
Warnings
- Succinylcholine should be administered only with great caution, if at all, prior to or during general anesthesia to patients receiving anticholinesterase medication because of possible respiratory or cardiovascular collapse.
- Caution should be observed in treating glaucoma with echothiophate iodide for ophthalmic solution in patients who are at the same time undergoing treatment with systemic anticholinesterase medications for myasthenia gravis, because of possible adverse additive effects.
Precautions
- 1. Gonioscopy is recommended prior to initiation of therapy. Routine examination to detect lens opacity should accompany clinical use of echothiophate iodide for ophthalmic solution.
- 2. Where there is a quiescent uveitis or a history of this condition, anticholinesterase therapy should be avoided or used cautiously because of the intense and persistent miosis and ciliary muscle contraction that may occur.
- 3. While systemic effects are infrequent, proper use of the drug requires digital compression of the nasolacrimal ducts for a minute or two following instillation to minimize drainage into the nasal chamber with its extensive absorption area. To prevent possible skin absorption, hands should be washed following instillation.
- 4. Temporary or permanent discontinuance of medication is necessary if cardiac irregularities occur.
- 5. Anticholinesterase drugs should be used with extreme caution, if at all, in patients with marked vagotonia, bronchial asthma, spastic gastrointestinal disturbances, peptic ulcer, pronounced bradycardia and hypotension, recent myocardial infarction, epilepsy, parkinsonism, and other disorders that may respond adversely to vagotonic effects.
- 6. Anticholinesterase drugs should be employed prior to ophthalmic surgery only as a considered risk because of the possible occurrence of hyphema.
- 7. Echothiophate iodide for ophthalmic solution should be used with great caution, if at all, where there is a prior history of retinal detachment.
- 8. Temporary discontinuance of medication is necessary if salivation, urinary incontinence, diarrhea, profuse sweating, muscle weakness, or respiratory difficulties occur.
- 9. Patients receiving echothiophate iodide for ophthalmic solution who are exposed to carbamate- or organophosphate-type insecticides and pesticides (professional gardeners, farmers, workers in plants manufacturing or formulating such products, etc.) should be warned of the additive systemic effects possible from absorption of the pesticide through the respiratory tract or skin. During periods of exposure to such pesticides, the wearing of respiratory masks, and frequent washing and clothing changes may be advisable.
Adverse Reactions
Clinical Trials Experience
- 1.Although the relationship, if any, of retinal detachment to the administration of echothiophate iodide for ophthalmic solution has not been established, retinal detachment has been reported in a few cases during the use of echothiophate iodide for ophthalmic solution in adult patients without a previous history of this disorder.
- 2. Stinging, burning, lacrimation, lid muscle twitching, conjunctival and ciliary redness, browache, induced myopia with visual blurring may occur.
- 3. Activation of latent iritis or uveitis may occur.
- 4. Iris cysts may form, and if treatment is continued, may enlarge and obscure vision. This occurrence is more frequent in children. The cysts usually shrink upon discontinuance of the medication, reduction in strength of the drops or frequency of instillation. Rarely, they may rupture or break free into the aqueous. Regular examinations are advisable when the drug is being prescribed for the treatment of accommodative esotropia.
- 5. Prolonged use may cause conjunctival thickening, obstruction of nasolacrimal canals.
- 6. Lens opacities occurring in patients under treatment for glaucoma with echothiophate iodide for ophthalmic solution have been reported and similar changes have been produced experimentally in normal monkeys. Routine examinations should accompany clinical use of the drug.
- 7. Paradoxical increase in intraocular pressure may follow anticholinesterase instillation. This may be alleviated by prescribing a sympathomimetic mydriatic such as phenylephrine.
- 8. Cardiac irregularities.
Postmarketing Experience
There is limited information regarding Postmarketing Experience of Echothiophate Iodide in the drug label.
Drug Interactions
- Echothiophate iodide for ophthalmic solution potentiates other cholinesterase inhibitors such as succinylcholine or organophosphate and carbamate insecticides. Patients undergoing systemic anticholinesterase treatment should be warned of the possible additive effects of echothiophate iodide for ophthalmic solution.
Use in Specific Populations
Pregnancy
- Teratogenic Effects
- Animal reproduction studies have not been conducted with echothiophate iodide for ophthalmic solution. It is also not known whether echothiophate iodide for ophthalmic solution can cause fetal harm when administered to a pregnant woman or can affect reproduction capacity. Echothiophate iodide for ophthalmic solution should be given to a pregnant woman only if clearly needed.
Pregnancy Category (AUS):
There is no Australian Drug Evaluation Committee (ADEC) guidance on usage of Echothiophate Iodide in women who are pregnant.
Labor and Delivery
There is no FDA guidance on use of Echothiophate Iodide during labor and delivery.
Nursing Mothers
- Because of the potential for serious adverse reactions in nursing infants from echothiophate iodide for ophthalmic solution, a decision should be made whether to discontinue nursing or to discontinue the drug, taking into account the importance of the drug to the mother.
Pediatric Use
- Safety and effectiveness in pediatric patients have been established.
Geriatic Use
- No overall differences in safety or effectiveness have been observed between elderly and younger patients.
Gender
There is no FDA guidance on the use of Echothiophate Iodide with respect to specific gender populations.
Race
There is no FDA guidance on the use of Echothiophate Iodide with respect to specific racial populations.
Renal Impairment
There is no FDA guidance on the use of Echothiophate Iodide in patients with renal impairment.
Hepatic Impairment
There is no FDA guidance on the use of Echothiophate Iodide in patients with hepatic impairment.
Females of Reproductive Potential and Males
There is no FDA guidance on the use of Echothiophate Iodide in women of reproductive potentials and males.
Immunocompromised Patients
There is no FDA guidance one the use of Echothiophate Iodide in patients who are immunocompromised.
Administration and Monitoring
Administration
- Ophthalmmic
Monitoring
There is limited information regarding Monitoring of Echothiophate Iodide in the drug label.
IV Compatibility
There is limited information regarding IV Compatibility of Echothiophate Iodide in the drug label.
Overdosage
There is limited information regarding Chronic Overdose of Echothiophate Iodide in the drug label.
Pharmacology
| |
| |
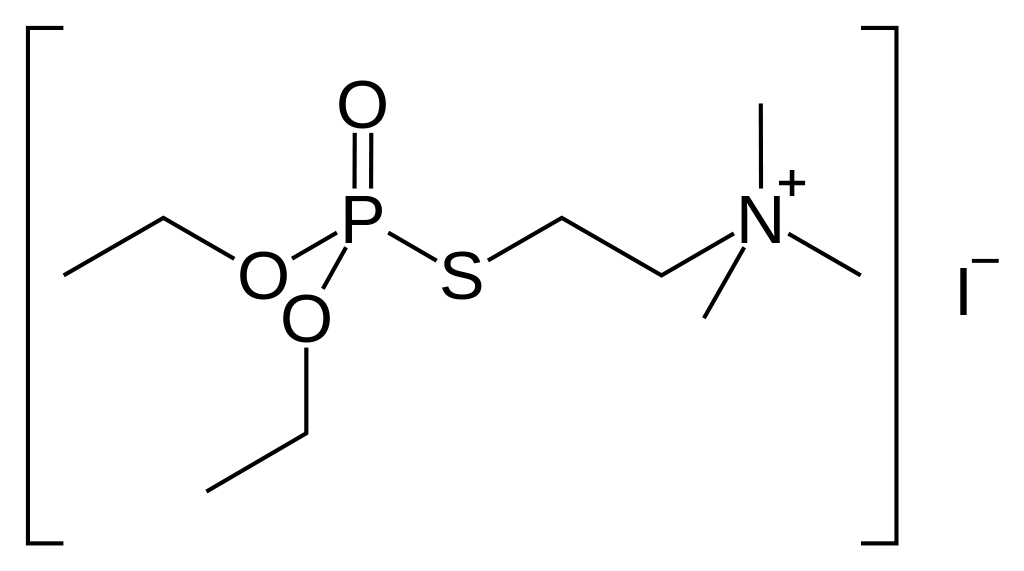
Echothiophate Iodide
| |
| Systematic (IUPAC) name | |
| 2-(Diethoxyphosphorylsulfanyl)ethyl-N,N,N-trimethylazanium iodide | |
| Identifiers | |
| CAS number | |
| ATC code | S01 |
| PubChem | |
| DrugBank | |
| Chemical data | |
| Formula | Template:OrganicBox atomTemplate:OrganicBox atomTemplate:OrganicBoxTemplate:OrganicBoxTemplate:OrganicBoxTemplate:OrganicBoxTemplate:OrganicBoxTemplate:OrganicBoxTemplate:OrganicBoxTemplate:OrganicBoxTemplate:OrganicBox atomTemplate:OrganicBoxTemplate:OrganicBoxTemplate:OrganicBox atomTemplate:OrganicBoxTemplate:OrganicBox atomTemplate:OrganicBox atomTemplate:OrganicBoxTemplate:OrganicBox atomTemplate:OrganicBoxTemplate:OrganicBoxTemplate:OrganicBoxTemplate:OrganicBox |
| Mol. mass | 383.228 g/mol |
| SMILES | & |
| Pharmacokinetic data | |
| Bioavailability | ? |
| Metabolism | ? |
| Half life | ? |
| Excretion | ? |
| Therapeutic considerations | |
| Pregnancy cat. |
? |
| Legal status | |
| Routes | Topical (eye drops) |
Mechanism of Action
- Echothiophate iodide for ophthalmic solution is a long-acting cholinesterase inhibitor for topical use which enhances the effect of endogenously liberated acetylcholine in iris, ciliary muscle, and other parasympathetically innervated structures of the eye. It thereby causes miosis, increase in facility of outflow of aqueous humor, fall in intraocular pressure, and potentiation of accommodation.
Structure
- Chemical name: (2-mercaptoethyl) trimethylammonium iodide O,O-diethyl phosphorothioate
- Structural formula
- Echothiophate iodide for ophthalmic solution occurs as a white, crystalline, water-soluble, hygroscopic solid having a slight mercaptan-like odor. When freeze-dried in the presence of potassium acetate, the mixture appears as a white amorphous deposit on the walls of the bottle.
- Each package contains materials for dispensing 5 mL of eyedrops: (1) bottle containing sterile echothiophate iodide for ophthalmic solution in one of four potencies [1.5 mg (0.03%), 3 mg (0.06%), 6.25 mg (0.125%), or 12.5 mg (0.25%)] as indicated on the label, with 40 mg potassium acetate in each case. Sodium hydroxide or acetic acid may have been incorporated to adjust pH during manufacturing. (2) a 5 mL bottle of sterile diluent containing chlorobutanol (chloral derivative), 0.55%; mannitol, 1.2%; boric acid, 0.06%; and sodium phosphate, 0.026%. (3) sterilized dropper.
Pharmacodynamics
There is limited information regarding Pharmacodynamics of Echothiophate Iodide in the drug label.
Pharmacokinetics
- Echothiophate iodide for ophthalmic solution will depress both plasma and erythrocyte cholinesterase levels in most patients after a few weeks of eyedrop therapy.
Nonclinical Toxicology
Carcinogenesis, Mutagenesis, Impairment of Fertility
- No data is available regarding carcinogenesis, mutagenesis, and impairment of fertility.
Clinical Studies
There is limited information regarding Clinical Studies of Echothiophate Iodide in the drug label.
How Supplied
- Each package contains sterile echothiophate iodide for ophthalmic solution, sterile diluent, and dropper for dispensing 5 mL eyedrops of the strength indicated on the label. Four potencies are available:
- NDC 0046-1062-05 . . . . . . . . . . . . . . . . . 1.5 mg package for 0.03%
- White amorphous deposit on bottle walls. Aluminum crimp seal is blue.
- NDC 0046-1064-05 . . . . . . . . . . . . . . . . . 3 mg package for 0.06%
- White amorphous deposit on bottle walls. Aluminum crimp seal is red.
- NDC 0046-1065-05 . . . . . . . . . . . . . . . . . 6.25 mg package for 0.125%
- White amorphous deposit on bottle walls. Aluminum crimp seal is green.
- NDC 0046-1066-05 . . . . . . . . . . . . . . . . . 12.5 mg package for 0.25%
- White amorphous deposit on bottle walls. Aluminum crimp seal is yellow.
- Prior to reconstitution: Store under refrigeration (2° to 8° C).
- After reconstitution: Store at room temperature (approximately 25° C). Do not refrigerate. Discard any unused solution after 4 weeks
Storage
There is limited information regarding Echothiophate Iodide Storage in the drug label.
Images
Drug Images
{{#ask: Page Name::Echothiophate Iodide |?Pill Name |?Drug Name |?Pill Ingred |?Pill Imprint |?Pill Dosage |?Pill Color |?Pill Shape |?Pill Size (mm) |?Pill Scoring |?NDC |?Drug Author |format=template |template=DrugPageImages |mainlabel=- |sort=Pill Name }}
Package and Label Display Panel
{{#ask: Label Page::Echothiophate Iodide |?Label Name |format=template |template=DrugLabelImages |mainlabel=- |sort=Label Page }}
Patient Counseling Information
There is limited information regarding Patient Counseling Information of Echothiophate Iodide in the drug label.
Precautions with Alcohol
- Alcohol-Echothiophate Iodide interaction has not been established. Talk to your doctor about the effects of taking alcohol with this medication.
Brand Names
- Phospholine Iodide
Look-Alike Drug Names
There is limited information regarding Echothiophate Iodide Look-Alike Drug Names in the drug label.
Drug Shortage Status
Price
References
The contents of this FDA label are provided by the National Library of Medicine.
{{#subobject:
|Label Page=Echothiophate Iodide |Label Name=Echothiophate Iodide04.png
}}
{{#subobject:
|Label Page=Echothiophate Iodide |Label Name=Echothiophate Iodide05.png
}}
{{#subobject:
|Label Page=Echothiophate Iodide |Label Name=Echothiophate Iodide06.png
}}
{{#subobject:
|Label Page=Echothiophate Iodide |Label Name=Echothiophate Iodide07.png
}}
