Polytetrafluoroethylene
Template:Chembox new Please Take Over This Page and Apply to be Editor-In-Chief for this topic: There can be one or more than one Editor-In-Chief. You may also apply to be an Associate Editor-In-Chief of one of the subtopics below. Please mail us [1] to indicate your interest in serving either as an Editor-In-Chief of the entire topic or as an Associate Editor-In-Chief for a subtopic. Please be sure to attach your CV and or biographical sketch.
In chemistry, polytetrafluoroethylene (PTFE) is a synthetic fluoropolymer which finds numerous applications. PTFE's most well known trademark in the industry is the DuPont brand name Teflon.
PTFE has an extremely low coefficient of friction and is used as a non-stick coating for pans and other cookware. It is very non-reactive, and so is often used in containers and pipework for reactive and corrosive chemicals. Where used as a lubricant, PTFE significantly reduces friction, wear and energy consumption of machinery.
History
The common statement that PTFE is a spin-off from the United States space program is an urban legend. PTFE was discovered accidentally by Roy Plunkett of DuPont in 1938.[1] While attempting to make a new CFC refrigerant, the perfluorethylene polymerized in its pressurized storage container. (In this original chemical reaction, iron from the inside of the container acted as a catalyst.) DuPont patented it in 1941 and registered the Teflon trademark in 1944.[2]
It was first sold commercially in 1946. By 1950, DuPont was producing over a million pounds (450 t) per year in Parkersburg, West Virginia. In 1954, French engineer Marc Grégoire created the first pan coated with Teflon non-stick resin.
An early advanced use was in the Manhattan Project as a material to coat valves and seals in the pipes holding highly reactive uranium hexafluoride in the vast uranium enrichment plant at Oak Ridge, Tennessee, when it was known as K416.
Properties
PTFE is a white solid at room temperature, with a density of about 2.2 g/cm³. According to DuPont its melting point is 327 °C (620.6 °F), but its properties degrade above 260 °C (500 °F).[3]
The coefficient of friction of plastics is usually measured against polished steel.[4] PTFE's coefficient of friction is 0.1 or less[3], which is the lowest of any known solid material. PTFE's resistance to van der Waals forces means that it is the only known surface to which a gecko cannot stick.[5]
PTFE has excellent dielectric properties. This is especially true at high radio frequencies, making it suitable for use as an insulator in cables and connector assemblies and as a material for printed circuit boards used at microwave frequencies. Combined with its high melting temperature, this makes it the material of choice as a high-performance substitute for the weaker and lower melting point polyethylene that is commonly used in low-cost applications. Its extremely high bulk resistivity makes it an ideal material for fabricating long life electrets, useful devices that are the electrostatic analogues of magnets.
Because of its chemical inertness, PTFE cannot be cross-linked like an elastomer. Therefore it has no "memory," and is subject to creep (also known as "cold flow" and "compression set"). This can be both good and bad. A little bit of creep allows PTFE seals to conform to mating surfaces better than most other plastic seals. Too much creep, however, and the seal is compromised. Compounding fillers control unwanted creep, as well as to improve wear, friction, and other properties. Sometimes metal springs apply continuous force to PTFE seals to give good contact, while permitting some creep.
Property values
| Property | Units | Value |
|---|---|---|
| Density | g/cm³ | 2.2 |
| Melting point | °C | 327 |
| Young's modulus | GPa | 0.5 |
| Yield strength | MPa | 23 |
| Coefficient of friction (measured against polished stainless steel) | 0.05-0.10 | |
| Dielectric constant | ε=2.1,tan(δ)<5(-4) | |
| Dielectric constant (60 Hz) | ε=2.1,tan(δ)<2(-4) | |
| Dielectric strength (1 MHz) | MV/m | 60 |
Applications
Due to its low friction, it is used for applications where sliding action of parts is needed: bearings, bushings, gears, slide plates, etc. In these applications it performs significantly better than nylon and acetal; it is comparable to ultra high-molecular weight polyethylene (UHMWPE), although UHMWPE is more resistant to wear than Teflon. For these applications, versions of teflon with mineral oil or molybdenum disulfide embedded as additional lubricants in its matrix are being manufactured.
Gore-Tex is a material incorporating fluoropolymer membrane with micropores. The roof of the Hubert H. Humphrey Metrodome in Minneapolis is one of the largest applications of Teflon PTFE coatings on Earth, using 20 acres (about 8 hectares) of the material in a double-layered, white dome, made with PTFE-coated fiberglass, that gives the stadium its distinctive appearance. The Millennium Dome in London is also substantially made of PTFE.
Powdered PTFE is used in pyrotechnic compositions as oxidizer together with powdered metals such as aluminum and magnesium. Upon ignition these mixtures form carbonaceous soot and the corresponding metal fluoride and release large amounts of heat. Hence they are used as infrared decoy flares and igniters for solid-fuel rocket propellants.[6]
PTFE is also used in body piercing, such as a sub-clavicle piercing, due to its flexibility and bio-compatibility.
In optical radiometry, sheets made from PTFE are used as measuring heads in spectroradiometers and broadband radiometers (e.g. illuminance meter and UV radiometer) due to its capability to diffuse a transmitting light nearly perfectly. Moreover, optical properties of PTFE stay constant over a wide range of wavelengths, from UV up to near infrared. In this region, the relation of its regular transmittance to diffuse transmittance is negligibly small so light transmitted through a diffuser (PTFE sheet) radiates like Lambert's cosine law. Thus, PTFE enables cosinusoidal angular response for a detector measuring the power of optical radiation at a surface, e.g., in solar irradiance measurements.
PTFE is also used to coat certain types of hardened, armor-piercing bullets, so as to reduce the amount of wear on the firearm's rifling. These are often mistakenly referred to as "cop-killer" bullets by virtue of PTFE's supposed ability to ease a bullet's passage through body armor.
Production
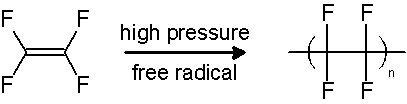
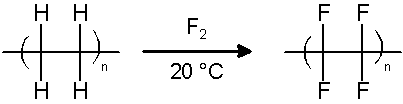
PTFE is either synthesized by the emulsion polymerization of tetrafluoroethylene monomer under pressure, using free-radical catalysts, or it may be produced by the direct substitution of hydrogen atoms on polyethylene with fluorine, using polyethylene and fluorine gas at 20 °C.[7]
Safety
While PTFE itself is chemically inert and non-toxic, it begins to deteriorate after the temperature of cookware reaches about 460 °F (237 °C), and decompose above 660 °F (350 °C). These degradation products can be lethal to birds, and can cause flu-like symptoms in humans.
By comparison, cooking fats, oils, and butter will begin to scorch and smoke at about 392 °F (200 °C), and meat is usually fried between 400–450 °F (200–230 °C), but empty cookware can exceed this temperature if left unattended on a hot burner.
A 1959 study, (conducted before the Food and Drug Administration approved the material for use in food processing equipment) showed that the toxicity of fumes given off by the coated pan on dry heating was less than that of fumes given off by ordinary cooking oils.[8]
Carcinogens in production
The United States Environmental Protection Agency's scientific advisory board found in 2005 that perfluorooctanoic acid (PFOA), a chemical compound used to make Teflon, is a "likely carcinogen." This finding was part of a draft report that has yet to be made final.[9] DuPont settled for $300 million in a 2004 lawsuit filed by residents near its manufacturing plant in Ohio and West Virginia based on groundwater pollution from this chemical. Currently this chemical is not regulated by the EPA.
In January 2006, DuPont, the only company that manufactures PFOA in the US, agreed to eliminate releases of the chemical from its manufacturing plants by 2015,[10] but did not commit to completely phasing out its use of the chemical. This agreement is said to apply to not only PTFE used in cookware but also other products such as food packaging, clothing, and carpeting. DuPont also stated that it cannot produce PTFE without the use of the chemical PFOA, although it is looking for a substitute.
PFOA is used only during the manufacture of the product—only a trace amount of PFOA remains after the curing process. DuPont maintains that there should be no measurable amount of PFOA on a finished pan, provided that it has been properly cured.[11]
Similar polymers
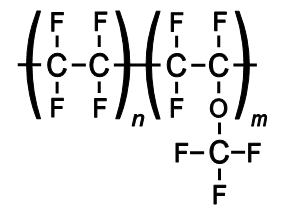
Other polymers with similar composition are also known by the Teflon name:
They retain the useful properties of PTFE of low friction and non-reactivity, but are more easily formable. FEP is softer than PTFE and melts at 260°C; it is highly transparent and resistant to sunlight.[12]
See also
Notes
- ↑ Roy J. Plunkett Chemical Heritage Foundation. Retrieved 10 September 2006.
- ↑ The story of Teflon
- ↑ 3.0 3.1 http://www2.dupont.com/Teflon_Industrial/en_US/tech_info/techinfo_compare.html Fluoropolymer Comparison - Typical Properties] www2.dupont.com. Retrieved 10 September 2006.
- ↑ Coefficient of Friction (COF) Testing of Plastics MatWeb Material Property Data Retrieved 1 January 2007.
- ↑ http://socrates.berkeley.edu/~peattiea/research_main.html
- ↑ E.-C. Koch "Metal-Fluorocarbon Pyrolants:III. Development and Application of Magnesium/Teflon/Viton" Propellants Explosives Pyrotechnics (2002),27(5),pp. 262-266.
- ↑ Mike Orthner, Polytetrafluoroethylene/"Teflon" Synthesis, accessed on 02 Oct 2006.
- ↑ Dale Blumenthal. "Is That Newfangled Cookware Safe?". Food and Drug Administration. Retrieved 2006-05-20.
- ↑ "Perfluorooctanoic Acid Human Health Risk Assessment Review Panel". Environmental Protection Agency. Retrieved 2005-05-20.
- ↑ Juliet Eilperin (2006-01-26). "Harmful PTFE Chemical To Be Eliminated by 2015". Washington Post. Retrieved 2006-09-10. Check date values in:
|date=(help) - ↑ "About Teflon". DuPont. Retrieved 2006-05-20.
- ↑ FEP Detailed Properties Parker-TexLoc, 13 April 2006. Retrieved 10 September 2006.
References
- Ellis, D.A.; Mabury, S.A.; Martin, J.W.; Muir, D.C.G. "Thermolysis of fluoropolymers as a potential source of halogenated organic acids in the environment." Nature 2001, 412 (6844), pp. 321-324.
External links
- The Chemical Heritage Foundation (2000). Roy J. Plunkett. Retrieved Oct 7, 2005.
- DuPont Teflon PTFE Material Data Sheet Retrieved Sept 6, 2006.
- DuPont (2005). Teflon News and Information. Retrieved Oct 7, 2005.
- PTFE vs FEP vs PFA vs ETFE vs PVDF The differences in Teflon tubing.
- DuPont (2005). Cooking Safety. Retrieved May 28, 2007.
- Environmental Working Group (2005). Canaries in the Kitchen - "Teflon Toxicosis" is deadly to pet birds. Are we at risk?. Retrieved Oct 7, 2005. More articles from EWG.
- Dr. Weil (2005-09-09). Teflon: The Sticky Business?
- Nature Publishing Group (2001-07-19). Thermolysis of fluoropolymers as a potential source of halogenated organic acids in the environment. Retrieved Oct 7, 2005.
- Organic Consumers Association / New York Times (2004-08-08). Dupont, Dupont Denies Poisoning Consumers with Teflon Products - Now in the Frying Pan. Retrieved Oct 7, 2005.
- Washington Post (2005-06-29 page A04). Compound in Teflon A 'Likely Carcinogen'. Retrieved Oct 7, 2005.
- CorpWatch / USA Today (2005-11-16). US: Engineer: DuPont hid facts about paper coating.
- CorpWatch / Associated Press (2005-11-29). US: EPA, DuPont in Settlement Over Chemical.
- CorpWatch / Associated Press (2005-12-14). US: DuPont fined more than $10M over Teflon.
- Los Angeles Times (2005-12-15). DuPont Settles Charges That It Hid Toxic Risk Data. Retrieved 2005-12-15.
- ABC News (2006-01-25). Government Moves to Curb Use of Chemical in Teflon.
- Washington Post (2006-01-26), Page A01. Harmful Teflon Chemical To Be Eliminated by 2015.
- Kopel, Dave (2004). Teflon Bullets. Retrieved Oct 7, 2005.
bg:Тефлон ca:Tefló cs:Polytetrafluorethylen da:Teflon de:Polytetrafluorethylen id:Teflon it:Politetrafluoroetilene he:טפלון lt:Teflonas nl:Teflon no:Polytetrafluoreten sk:Polytetrafluóretylén fi:Teflon sv:Teflon