Diazepam (rectal)
Editor-In-Chief: C. Michael Gibson, M.S., M.D. [1]; Associate Editor(s)-in-Chief: Ammu Susheela, M.D. [2]
Disclaimer
WikiDoc MAKES NO GUARANTEE OF VALIDITY. WikiDoc is not a professional health care provider, nor is it a suitable replacement for a licensed healthcare provider. WikiDoc is intended to be an educational tool, not a tool for any form of healthcare delivery. The educational content on WikiDoc drug pages is based upon the FDA package insert, National Library of Medicine content and practice guidelines / consensus statements. WikiDoc does not promote the administration of any medication or device that is not consistent with its labeling. Please read our full disclaimer here.
Overview
Diazepam (rectal) is an general anesthetic that is FDA approved for the treatment of refractory, patients with epilepsy, on stable regimens of AEDs. Common adverse reactions include hypotension, rash, diarrhea, muscle weakness, ataxia, incoordination, somnolence, euphoria, respiratory depression, and fatigue.
Adult Indications and Dosage
FDA-Labeled Indications and Dosage (Adult)
Indication
- Diazepam rectal gel is a gel formulation of diazepam intended for rectal administration in the management of selected, refractory, patients with epilepsy on stable regimens of AEDs, who require intermittent use of diazepam to control bouts of increased seizure activity.
- Evidence to support the use of diazepam rectal gel was adduced in two controlled trials that enrolled patients with partial onset or generalized convulsive seizures who were identified jointly by their caregivers and physicians as suffering intermittent and periodic episodes of markedly increased seizure activity, sometimes heralded by nonconvulsive symptoms, that for the individual patient were characteristic and were deemed by the prescriber to be of a kind for which a benzodiazepine would ordinarily be administered acutely. Although these clusters or bouts of seizures differed among patients, for any individual patient the clusters of seizure activity were not only stereotypic but were judged by those conducting and participating in these studies to be distinguishable from other seizures suffered by that patient. The conclusion that a patient experienced such unique episodes of seizure activity was based on historical information.
Dosage
- This section is intended primarily for the prescriber; however, the prescriber should also be aware of the dosing information and directions for use provided in the patient package insert.
- A decision to prescribe Diazepam rectal gel involves more than the diagnosis and the selection of the correct dose for the patient.
- First, the prescriber must be convinced from historical reports and/or personal observations that the patient exhibits the characteristic identifiable seizure cluster that can be distinguished from the patient¹s usual seizure activity by the caregiver who will be responsible for administering Diazepam rectal gel.
- Second, because Diazepam rectal gel is only intended for adjunctive use, the prescriber must ensure that the patient is receiving an optimal regimen of standard anti-epileptic drug treatment and is, nevertheless, continuing to experience these characteristic episodes.
- Third, because a non-health professional will be obliged to identify episodes suitable for treatment, make the decision to administer treatment upon that identification, administer the drug, monitor the patient, and assess the adequacy of the response to treatment, a major component of the prescribing process involves the necessary instruction of this individual.
- Fourth, the prescriber and caregiver must have a common understanding of what is and is not an episode of seizures that is appropriate for treatment, the timing of administration in relation to the onset of the episode, the mechanics of administering the drug, how and what to observe following administration, and what would constitute an outcome requiring immediate and direct medical attention.
Calculating Prescribed Dose
- The Diazepam rectal gel dose should be individualized for maximum beneficial effect. The recommended dose of Diazepam rectal gel is 0.2-0.5 mg/kg depending on age. See the dosing table for specific recommendations.
Off-Label Use and Dosage (Adult)
Guideline-Supported Use
Anesthesia; Adjunct.
- Benzodiazepine withdrawal.
- Sedation for a mechanically ventilated patient, Intensive care unit.
Non–Guideline-Supported Use
There is limited information regarding Off-Label Non–Guideline-Supported Use of Diazepam (rectal) in adult patients.
Pediatric Indications and Dosage
FDA-Labeled Indications and Dosage (Pediatric)
There is limited information regarding FDA-Labeled Use of Diazepam (rectal) in pediatric patients.
Off-Label Use and Dosage (Pediatric)
Guideline-Supported Use
There is limited information regarding Off-Label Guideline-Supported Use of Diazepam (rectal) in pediatric patients.
Non–Guideline-Supported Use
There is limited information regarding Off-Label Non–Guideline-Supported Use of Diazepam (rectal) in pediatric patients.
Contraindications
- Diazepam rectal gel is contraindicated in patients with a known hypersensitivity to diazepam. Diazepam rectal gel may be used in patients with open angle glaucoma who are receiving appropriate therapy but is contraindicated in acute narrow angle glaucoma.
Warnings
General
- Diazepam rectal gel should only be administered by caregivers who in the opinion of the prescribing physician 1) are able to distinguish the distinct cluster of seizures (and/or the events presumed to herald their onset) from the patient’s ordinary seizure activity, 2) have been instructed and judged to be competent to administer the treatment rectally, 3) understand explicitly which seizure manifestations may or may not be treated with diazepam rectal gel, and 4) are able to monitor the clinical response and recognize when that response is such that immediate professional medical evaluation is required.
CNS Depression
- Because diazepam rectal gel produces CNS depression, patients receiving this drug who are otherwise capable and qualified to do so should be cautioned against engaging in hazardous occupations requiring mental alertness, such as operating machinery, driving a motor vehicle, or riding a bicycle until they have completely returned to their level of baseline functioning.
- Although diazepam rectal gel is indicated for use solely on an intermittent basis, the potential for a synergistic CNS-depressant effect when used simultaneously with alcohol or other CNS depressants must be considered by the prescribing physician, and appropriate recommendations made to the patient and/or caregiver.
- Prolonged CNS depression has been observed in neonates treated with diazepam. Therefore, diazepam rectal gel is not recommended for use in children under six months of age.
Pregnancy Risks
- No clinical studies have been conducted with diazepam rectal gel in pregnant women. Data from several sources raise concerns about the use of diazepam during pregnancy.
- Animal Findings: Diazepam has been shown to be teratogenic in mice and hamsters when given orally at single doses of 100 mg/kg or greater (approximately eight times the maximum recommended human dose [MRHD=1 mg/kg/day] or greater on a mg/m2 basis).
- Cleft palate and exencephaly are the most common and consistently reported malformations produced in these species by administration of high, maternally-toxic doses of diazepam during organogenesis. Rodent studies have indicated that prenatal exposure to diazepam doses similar to those used clinically can produce longterm changes in cellular immune responses, brain neurochemistry, and behavior.
- General Concerns and Considerations About Anticonvulsants: Reports suggest an association between the use of anticonvulsant drugs by women with epilepsy and an elevated incidence of birth defects in children born to these women. Data are more extensive with respect to phenytoin and phenobarbital, but a smaller number of systematic or anecdotal reports suggest a possible similar association with the use of all known anticonvulsant drugs.
- The reports suggesting an elevated incidence of birth defects in children of drug treated epileptic women cannot be regarded as adequate to prove a definite cause and effect relationship. There are intrinsic methodologic problems in obtaining adequate data on drug teratogenicity in humans; the possibility also exists that other factors, e.g., genetic factors or the epileptic condition itself, may be more important than drug therapy in leading to birth defects. The great majority of mothers on anticonvulsant medication deliver normal infants. It is important to note that anticonvulsant drugs should not be discontinued in patients in whom the drug is administered to prevent seizures because of the strong possibility of precipitating status epilepticus with attendant hypoxia and threat to life. In individual cases where the severity and frequency of the seizure disorder are such that the removal of medication does not pose a serious threat to the patient, discontinuation of the drug may be considered prior to and during pregnancy, although it cannot be said with any confidence that even mild seizures do not pose some hazards to the developing embryo or fetus.
- General Concerns About Benzodiazepines: An increased risk of congenital malformations associated with the use of benzodiazepine drugs has been suggested in several studies.
- There may also be non-teratogenic risks associated with the use of benzodiazepines during pregnancy. There have been reports of neonatal flaccidity, respiratory and feeding difficulties, and hypothermia in children born to mothers who have been receiving benzodiazepines late in pregnancy. In addition, children born to mothers receiving benzodiazepines on a regular basis late in pregnancy may be at some risk of experiencing withdrawal symptoms during the postnatal period.
- Advice Regarding the Use of Diazepam rectal gel in Women of Childbearing Potential: In general, the use of Diazepam rectal gel in women of childbearing potential, and more specifically during known pregnancy, should be considered only when the clinical situation warrants the risk to the fetus.
- The specific considerations addressed above regarding the use of anticonvulsants in epileptic women of childbearing potential should be weighed in treating or counseling these women.
- Because of experience with other members of the benzodiazepine class, Diazepam rectal gel is assumed to be capable of causing an increased risk of congenital abnormalities when administered to a pregnant woman during the first trimester. The possibility that a woman of childbearing potential may be pregnant at the time of institution of therapy should be considered. If this drug is used during pregnancy, or if the patient becomes pregnant while taking this drug, the patient should be apprised of the potential hazard to the fetus. Patients should also be advised that if they become pregnant during therapy or intend to become pregnant they should communicate with their physician about the desirability of discontinuing the drug.
Withdrawal Symptoms
- Withdrawal symptoms of the barbiturate type have occurred after the discontinuation of regular use of benzodiazepines.
Chronic Use
- Diazepam rectal gel is not recommended for chronic, daily use as an anticonvulsant because of the potential for development of tolerance to diazepam. * Chronic daily use of diazepam may increase the frequency and/or severity of tonic clonic seizures, requiring an increase in the dosage of standard anticonvulsant medication. In such cases, abrupt withdrawal of chronic diazepam may also be associated with a temporary increase in the frequency and/or severity of seizures.
Use in Patients with Petit Mal Status
- Tonic status epilepticus has been precipitated in patients treated with IV diazepam for petit mal status or petit mal variant status.
Adverse Reactions
Clinical Trials Experience
- Diazepam rectal gel adverse event data were collected from double-blind, placebo-controlled studies and open-label studies. The majority of adverse events were mild to moderate in severity and transient in nature.
- Two patients who received Diazepam rectal gel died seven to 15 weeks following treatment; neither of these deaths was deemed related to Diazepam rectal gel.
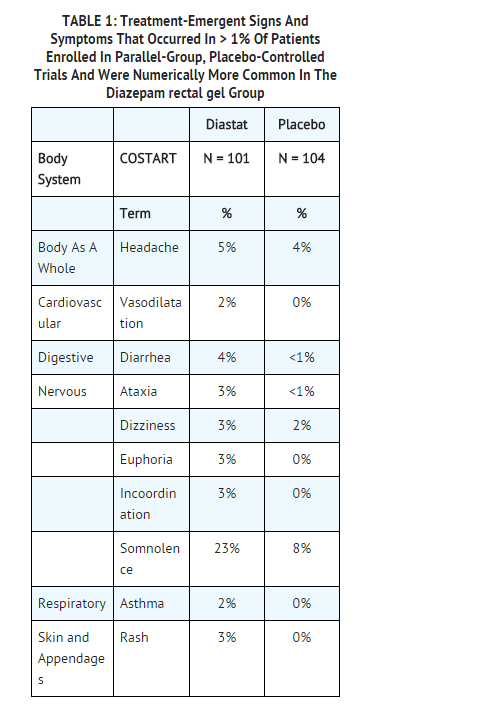
- The most frequent adverse event reported to be related to Diazepam rectal gel in the two double-blind, placebo-controlled studies was somnolence (23%). Less frequent adverse events were dizziness, headache, pain, abdominal pain, nervousness, vasodilatation, diarrhea, ataxia, euphoria, incoordination, asthma, rhinitis, and rash, which occurred in approximately 2-5% of patients.
- Approximately 1.4% of the 573 patients who received Diazepam rectal gel in clinical trials of epilepsy discontinued treatment because of an adverse event. The adverse event most frequently associated with discontinuation (occurring in three patients) was somnolence. Other adverse events most commonly associated with discontinuation and occurring in two patients were hypoventilation and rash. Adverse events occurring in one patient were asthenia, hyperkinesia, incoordination, vasodilatation and urticaria. These events were judged to be related to diazepam rectal gel.
- In the two domestic double-blind, placebo-controlled, parallel-group studies, the proportion of patients who discontinued treatment because of adverse events was 2% for the group treated with Diazepam rectal gel, versus 2% for the placebo group. In the Diazepam rectal gel group, the adverse events considered the primary reason for discontinuation were different in the two patients who discontinued treatment; one discontinued due to rash and one discontinued due to lethargy. The primary reason for discontinuation in the patients treated with placebo was lack of effect.
Adverse Event Incidence in Controlled Clinical Trials
- Table 1 lists treatment-emergent signs and symptoms that occurred in > 1% of patients enrolled in parallel-group, placebo-controlled trials and were numerically more common in the Diazepam rectal gel group. Adverse events were usually mild or moderate in intensity.
- The prescriber should be aware that these figures, obtained when Diazepam rectal gel was added to concurrent antiepileptic drug therapy, cannot be used to predict the frequency of adverse events in the course of usual medical practice when patient characteristics and other factors may differ from those prevailing during clinical studies. Similarly, the cited frequencies cannot be directly compared with figures obtained from other clinical investigations involving different treatments, uses, or investigators. An inspection of these frequencies, however, does provide the prescribing physician with one basis to estimate the relative contribution of drug and non-drug factors to the adverse event incidences in the population studied.
- Other events reported by 1% or more of patients treated in controlled trials but equally or more frequent in the placebo group than in the Diazepam rectal gel group were abdominal pain, pain, nervousness, and rhinitis. Other events reported by fewer than 1% of patients were infection, anorexia, vomiting, anemia, lymphadenopathy, grand mal convulsion, hyperkinesia, cough increased, pruritus, sweating, mydriasis, and urinary tract infection.
- The pattern of adverse events was similar for different age, race and gender groups.
Other Adverse Events Observed During All Clinical Trials
- Diazepam rectal gel has been administered to 573 patients with epilepsy during all clinical trials, only some of which were placebo-controlled. During these trials, all adverse events were recorded by the clinical investigators using terminology of their own choosing. To provide a meaningful estimate of the proportion of individuals having adverse events, similar types of events were grouped into a smaller number of standardized categories using modified COSTART dictionary terminology. These categories are used in the listing below. All of the events listed below occurred in at least 1% of the 573 individuals exposed to Diazepam rectal gel.
- All reported events are included except those already listed above, events unlikely to be drug-related, and those too general to be informative. Events are included without regard to determination of a causal relationship to diazepam.
- BODY AS A WHOLE: Asthenia
- CARDIOVASCULAR: Hypotension, vasodilatation
- NERVOUS: Agitation, confusion, convulsion, dysarthria, emotional lability, speech disorder, thinking abnormal, vertigo
- RESPIRATORY: Hiccup
- The following infrequent adverse events were not seen with Diazepam rectal gel but have been reported previously with diazepam use: depression, slurred speech, syncope, constipation, changes in libido, urinary retention, bradycardia, cardiovascular collapse, nystagmus, urticaria, neutropenia and jaundice.
- Paradoxical reactions such as acute hyperexcited states, anxiety, hallucinations, increased muscle spasticity, insomnia, rage, sleep disturbances and stimulation have been reported with diazepam; should these occur, use of Diazepam rectal gel should be discontinued.
Postmarketing Experience
There is limited information regarding Postmarketing Experience of Diazepam (rectal) in the drug label.
Drug Interactions
- If Diazepam rectal gel is to be combined with other psychotropic agents or other CNS depressants, careful consideration should be given to the pharmacology of the agents to be employed particularly with known compounds which may potentiate the action of diazepam, such as phenothiazines, narcotics, barbiturates, MAO inhibitors and other antidepressants.
- The clearance of diazepam and certain other benzodiazepines can be delayed in association with cimetidine administration. The clinical significance of this is unclear.
- Valproate may potentiate the CNS-depressant effects of diazepam.
- There have been no clinical studies or reports in literature to evaluate the interaction of rectally administered diazepam with other drugs. As with all drugs, the potential for interaction by a variety of mechanisms is a possibility.
- Effect of Other Drugs on Diazepam Metabolism: In vitro studies using human liver preparations suggest that CYP2C19 and CYP3A4 are the principal isozymes involved in the initial oxidative metabolism of diazepam. Therefore, potential interactions may occur when diazepam is given concurrently with agents that affect CYP2C19 and CYP3A4 activity. Potential inhibitors of CYP2C19 (e.g., cimetidine, quinidine, and tranylcypromine) and CYP3A4 (e.g., ketoconazole, troleandomycin, and clotrimazole) could decrease the rate of diazepam elimination, while inducers of CYP2C19 (e.g., rifampin) and CYP3A4 (e.g., carbamazepine, phenytoin, dexamethasone and phenobarbital) could increase the rate of elimination of diazepam.
- Effect of Diazepam on the Metabolism of Other Drugs: There are no reports as to which isozymes could be inhibited or induced by diazepam. But, based on the fact that diazepam is a substrate for CYP2C19 and CYP3A4, it is possible that diazepam may interfere with the metabolism of drugs which are substrates for CYP2C19, (e.g. omeprazole, propranolol, and imipramine) and CYP3A4 (e.g. cyclosporine, paclitaxel, terfenadine, theophylline, and warfarin) leading to a potential drug-drug interaction.
Carcinogenesis, Mutagenesis, Impairment of Fertility
- The carcinogenic potential of rectal diazepam has not been evaluated. In studies in which mice and rats were administered diazepam in the diet at a dose of 75 mg/kg/day (approximately six and 12 times, respectively, the maximum recommended human dose [MRHD=1 mg/kg/day] on a mg/m2 basis) for 80 and 104 weeks, respectively, an increased incidence of liver tumors was observed in males of both species.
- The data currently available are inadequate to determine the mutagenic potential of diazepam.
- Reproduction studies in rats showed decreases in the number of pregnancies and in the number of surviving offspring following administration of an oral dose of 100 mg/kg/day (approximately 16 times the MRHD on a mg/m2 basis) prior to and during mating and throughout gestation and lactation. No adverse effects on fertility or offspring viability were noted at a dose of 80 mg/kg/day (approximately 13 times the MRHD on a mg/m2 basis).
Use in Specific Populations
Pregnancy
- Pregnancy Category
- Australian Drug Evaluation Committee (ADEC) Pregnancy Category
There is no Australian Drug Evaluation Committee (ADEC) guidance on usage of Diazepam (rectal) in women who are pregnant.
Labor and Delivery
- In humans, measurable amounts of diazepam have been found in maternal and cord blood, indicating placental transfer of the drug. Until additional information is available, Diazepam rectal gel is not recommended for obstetrical use.
Nursing Mothers
- Because diazepam and its metabolites may be present in human breast milk for prolonged periods of time after acute use of Diazepam rectal gel, patients should be advised not to breast-feed for an appropriate period of time after receiving treatment with Diazepam rectal gel.
Pediatric Use
There is no FDA guidance on the use of Diazepam (rectal) with respect to pediatric patients.
Geriatic Use
There is no FDA guidance on the use of Diazepam (rectal) with respect to geriatric patients.
Gender
There is no FDA guidance on the use of Diazepam (rectal) with respect to specific gender populations.
Race
There is no FDA guidance on the use of Diazepam (rectal) with respect to specific racial populations.
Renal Impairment
There is no FDA guidance on the use of Diazepam (rectal) in patients with renal impairment.
Hepatic Impairment
There is no FDA guidance on the use of Diazepam (rectal) in patients with hepatic impairment.
Females of Reproductive Potential and Males
There is no FDA guidance on the use of Diazepam (rectal) in women of reproductive potentials and males.
Immunocompromised Patients
There is no FDA guidance one the use of Diazepam (rectal) in patients who are immunocompromised.
Administration and Monitoring
Administration
Monitoring
There is limited information regarding Monitoring of Diazepam (rectal) in the drug label.
IV Compatibility
There is limited information regarding IV Compatibility of Diazepam (rectal) in the drug label.
Overdosage
Two patients in the clinical studies received more than twice the target dose; no adverse events were reported.
Previous reports of diazepam overdosage have shown that manifestations of diazepam overdosage include somnolence, confusion, coma, and diminished reflexes. Respiration, pulse and blood pressure should be monitored, as in all cases of drug overdosage, although, in general, these effects have been minimal. General supportive measures should be employed, along with intravenous fluids, and an adequate airway maintained. Hypotension may be combated by the use of levarterenol or metaraminol. Dialysis is of limited value.
Flumazenil, a specific benzodiazepine-receptor antagonist, is indicated for the complete or partial reversal of the sedative effects of benzodiazepines and may be used in situations when an overdose with a benzodiazepine is known or suspected. Prior to the administration of flumazenil, necessary measures should be instituted to secure airway, ventilation and intravenous access. Flumazenil is intended as an adjunct to, not as a substitute for, proper management of benzodiazepine overdose. Patients treated with flumazenil should be monitored for resedation, respiratory depression and other residual benzodiazepine effects for an appropriate period after treatment. The prescriber should be aware of a risk of seizure in association with flumazenil treatment, particularly in long-term benzodiazepine users and in cyclic antidepressant overdose.
Pharmacology
Mechanism of Action
- * Diazepam is a benzodiazepine that exerts anxiolytic, sedative, muscle-relaxant, anticonvulsant and amnestic effects. Most of these effects are thought to result from a facilitation of the action of gamma aminobutyric acid (GABA), an inhibitory neurotransmitter in the central nervous system.
Structure
- Diazepam rectal gel rectal delivery system is a non-sterile diazepam gel provided in a prefilled, unit-dose, rectal delivery system. Diazepam rectal gel contains 5 mg/mL diazepam, propylene glycol, ethyl alcohol (10%), hydroxypropyl methylcellulose, sodium benzoate, benzyl alcohol (1.5%), benzoic acid and water. Diazepam rectal gel is clear to slightly yellow and has a pH between 6.5 -7.2.
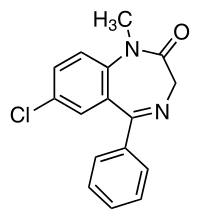
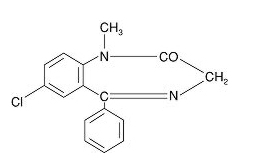
- Diazepam, the active ingredient of diazepam rectal gel, is a benzodiazepine anticonvulsant with the chemical name 7-chloro-1,3-dihydro-1-methyl-5-phenyl-2H-1,4-benzodiazepin-2-one. The structural formula is as follows:
Pharmacodynamics
There is limited information regarding Pharmacodynamics of Diazepam (rectal) in the drug label.
Pharmacokinetics
- Pharmacokinetic information of diazepam following rectal administration was obtained from studies conducted in healthy adult subjects. No pharmacokinetic studies were conducted in pediatric patients. Therefore, information from the literature is used to define pharmacokinetic labeling in the pediatric population.
- Diazepam rectal gel is well absorbed following rectal administration, reaching peak plasma concentrations in 1.5 hours. The absolute bioavailability of Diazepam rectal gel relative to Valium® injectable is 90%. The volume of distribution of Diazepam rectal gel is calculated to be approximately 1 L/kg. The mean elimination half-life of diazepam and desmethyldiazepam following administration of a 15 mg dose of Diazepam rectal gel was found to be about 46 hours (CV=43%) and 71 hours (CV=37%), respectively. Both diazepam and its major active metabolite desmethyldiazepam bind extensively to plasma proteins (95-98%).
Nonclinical Toxicology
There is limited information regarding Nonclinical Toxicology of Diazepam (rectal) in the drug label.
Clinical Studies
There is limited information regarding Clinical Studies of Diazepam (rectal) in the drug label.
How Supplied
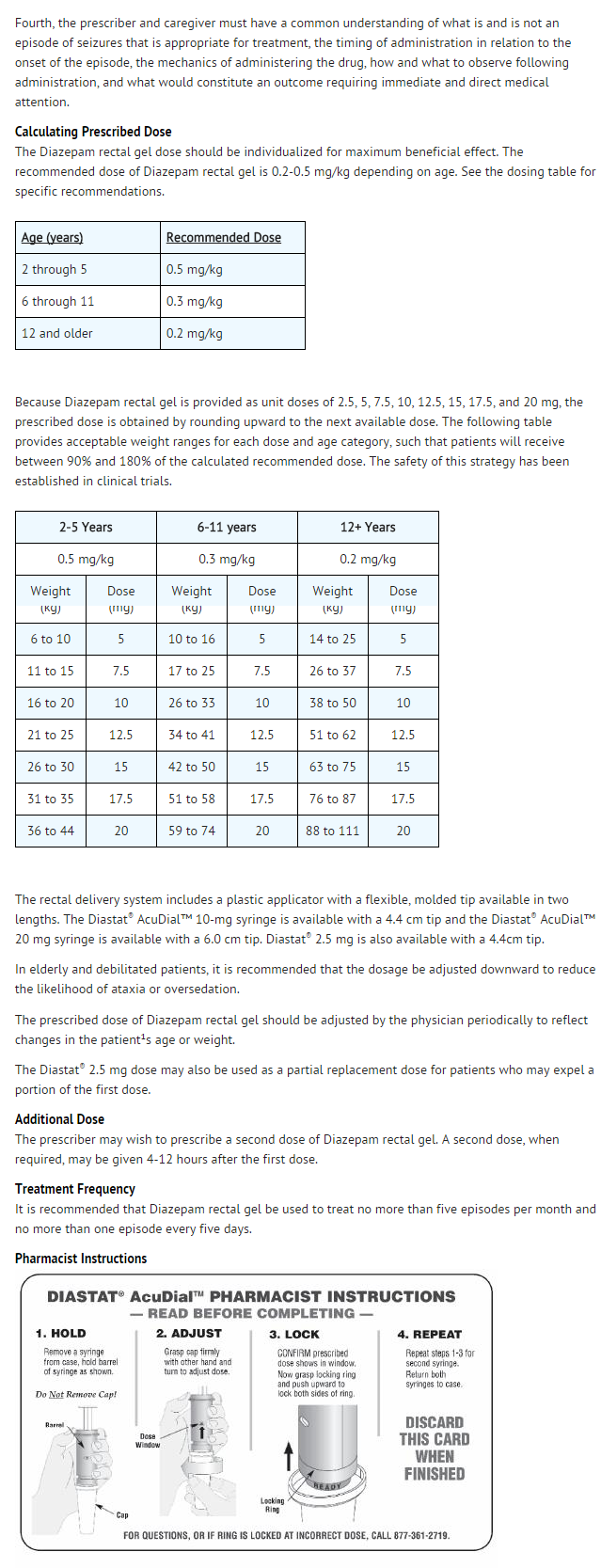
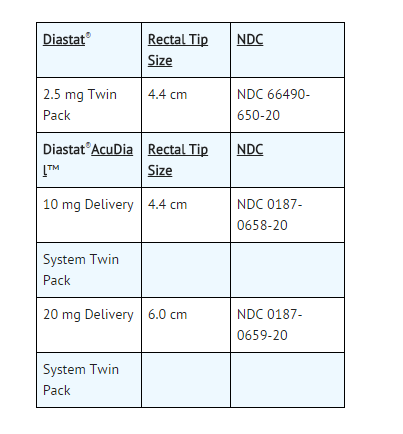
- Diazepam rectal gel rectal delivery system is a non-sterile, prefilled, unit dose, rectal delivery system. The rectal delivery system includes a plastic applicator with a flexible, molded tip available in two lengths, designated for convenience as 10 mg * Delivery System and 20 mg Delivery System. The available doses from 20 mg delivery system are 12.5 mg, 15 mg, 17.5 mg and 20 mg. The available doses from 10 mg delivery system are 5 mg, 7.5 mg and 10 mg. The Diazepam rectal gel delivery system is available in the following three presentations:
Storage
- Store at 25°C (77°F); excursions permitted to 15-30°C (59-86°F)
Images
Drug Images
{{#ask: Page Name::Diazepam (rectal) |?Pill Name |?Drug Name |?Pill Ingred |?Pill Imprint |?Pill Dosage |?Pill Color |?Pill Shape |?Pill Size (mm) |?Pill Scoring |?NDC |?Drug Author |format=template |template=DrugPageImages |mainlabel=- |sort=Pill Name }}
Package and Label Display Panel


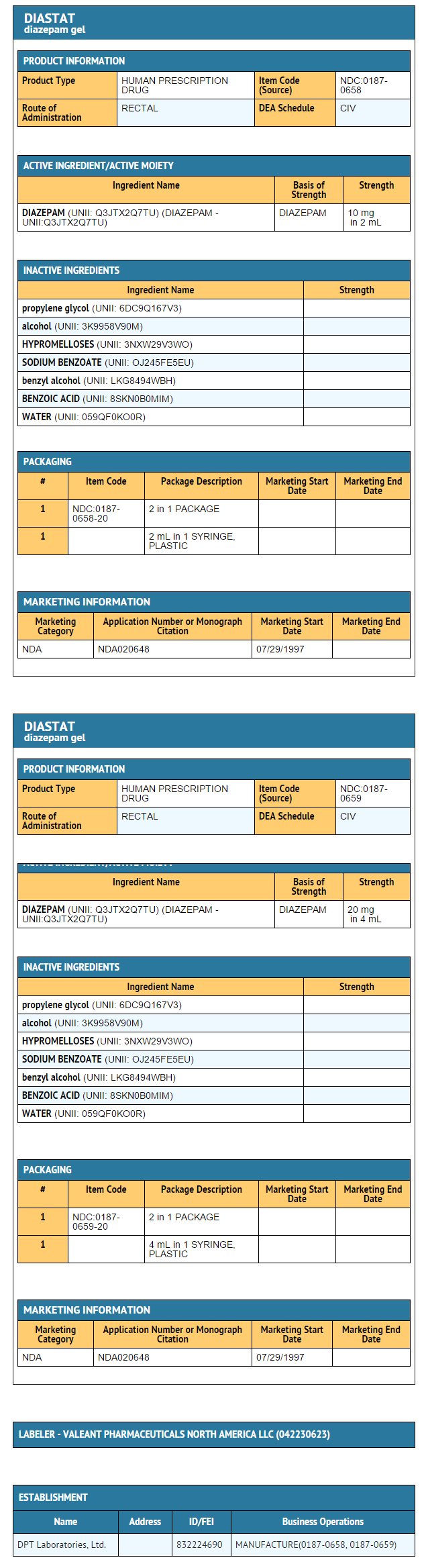
{{#ask: Label Page::Diazepam (rectal) |?Label Name |format=template |template=DrugLabelImages |mainlabel=- |sort=Label Page }}
Patient Counseling Information
There is limited information regarding Patient Counseling Information of Diazepam (rectal) in the drug label.
Precautions with Alcohol
- Concomitant use with alcohol is not recommended due to enhancement of the sedative effect.
Brand Names
- DIASTAT®[1]
Look-Alike Drug Names
There is limited information regarding Diazepam (rectal) Look-Alike Drug Names in the drug label.
Drug Shortage Status
Price
References
The contents of this FDA label are provided by the National Library of Medicine.