Deutetrabenazine
Editor-In-Chief: C. Michael Gibson, M.S., M.D. [1]; Associate Editor(s)-in-Chief: Sonya Gelfand, Anmol Pitliya, M.B.B.S. M.D.[2]
Disclaimer
WikiDoc MAKES NO GUARANTEE OF VALIDITY. WikiDoc is not a professional health care provider, nor is it a suitable replacement for a licensed healthcare provider. WikiDoc is intended to be an educational tool, not a tool for any form of healthcare delivery. The educational content on WikiDoc drug pages is based upon the FDA package insert, National Library of Medicine content and practice guidelines / consensus statements. WikiDoc does not promote the administration of any medication or device that is not consistent with its labeling. Please read our full disclaimer here.
Black Box Warning
|
WARNING: DEPRESSION AND SUICIDALITY IN PATIENTS WITH HUNTINGTON’S DISEASE
See full prescribing information for complete Boxed Warning.
|
Overview
Deutetrabenazine is a vesicular monoamine transporter 2 (VMAT2) inhibitor that is FDA approved for the treatment of chorea associated with Huntington’s disease, and tardive dyskinesia. There is a Black Box Warning for this drug as shown here. Common adverse reactions include somnolence, diarrhea, dry mouth, fatigue, nasopharyngitis, and insomnia.
Adult Indications and Dosage
FDA-Labeled Indications and Dosage (Adult)
Indications
- Deutetrabenazine is indicated for the treatment of:
- Chorea associated with Huntington’s disease
- Tardive dyskinesia in adults
Dosing Information
- The dose of deutetrabenazine is determined individually for each patient based on reduction of chorea or tardive dyskinesia and tolerability. When first prescribed to patients who are not being switched from tetrabenazine (a related VMAT2 inhibitor), the recommended starting dose of deutetrabenazine is 6 mg administered orally once daily for patients with Huntington’s disease and 12 mg per day (6 mg twice daily) for patients with tardive dyskinesia.
- The dose of deutetrabenazine may be increased at weekly intervals in increments of 6 mg per day to a maximum recommended daily dosage of 48 mg.
- Administer total daily dosages of 12 mg or above in two divided doses.
- Administer deutetrabenazine with food.
- Swallow deutetrabenazine whole. Do not chew, crush, or break tablets.
- For patients at risk for QT prolongation, assess the QT interval before and after increasing total deutetrabenazine dosage above 24 mg per day.
Switching Patients from Tetrabenazine to Deutetrabenzine
- Discontinue tetrabenazine and initiate deutetrabenazine the following day. The recommended initial dosing regimen of deutetrabenazine in patients switching from tetrabenazine to deutetrabenazine is shown in Table 1.

- After patients are switched to deutetrabenazine, the dose may be adjusted at weekly intervals
Dosage Adjustment with Strong CYP2D6 Inhibitors
- In patients receiving strong CYP2D6 inhibitors (e.g., quinidine, antidepressants such as paroxetine, fluoxetine, and bupropion), the total daily dosage of deutetrabenazine should not exceed 36 mg (maximum single dose of 18 mg).
Dosage Adjustment in Poor CYP2D6 Metabolizers
- In patients who are poor CYP2D6 metabolizers, the total daily dosage of deutetrabenazine should not exceed 36 mg (maximum single dose of 18 mg).
Discontinuation and Interruption of Treatment
- Treatment with deutetrabenazine can be discontinued without tapering. Following treatment interruption of greater than one week, deutetrabenazine therapy should be re-titrated when resumed. For treatment interruption of less than one week, treatment can be resumed at the previous maintenance dose without titration.
Off-Label Use and Dosage (Adult)
Guideline-Supported Use
There is limited information regarding deutetrabenazine Off-Label Guideline-Supported Use and Dosage (Adult) in the drug label.
Non–Guideline-Supported Use
There is limited information regarding deutetrabenazine Off-Label Non-Guideline-Supported Use and Dosage (Adult) in the drug label.
Pediatric Indications and Dosage
FDA-Labeled Indications and Dosage (Pediatric)
There is limited information regarding Deutetrabenazine FDA-Labeled Indications and Dosage (Pediatric) in the drug label.
Off-Label Use and Dosage (Pediatric)
Guideline-Supported Use
There is limited information regarding deutetrabenazine Off-Label Guideline-Supported Use and Dosage (Pediatric) in the drug label.
Non–Guideline-Supported Use
There is limited information regarding deutetrabenazine Off-Label Non-Guideline-Supported Use and Dosage (Pediatric) in the drug label.
Contraindications
- Deutetrabenazine is contraindicated in patients:
- With Huntington’s disease who are suicidal, or have untreated or inadequately treated depression.
- With hepatic impairment.
- Taking reserpine. At least 20 days should elapse after stopping reserpine before starting deutetrabenazine.
- Taking monoamine oxidase inhibitors (MAOIs). Deutetrabenazine should not be used in combination with an MAOI, or within 14 days of discontinuing therapy with an MAOI.
- Taking tetrabenazine (XENAZINE®) or valbenazine.
Warnings
|
WARNING: DEPRESSION AND SUICIDALITY IN PATIENTS WITH HUNTINGTON’S DISEASE
See full prescribing information for complete Boxed Warning.
|
Depression and Suicidality in Patients with Huntington’s Disease
- Patients with Huntington’s disease are at increased risk for depression, and suicidal ideation or behaviors (suicidality). Deutetrabenazine may increase the risk for suicidality in patients with Huntington’s disease.
- In a 12-week, double-blind, placebo-controlled trial, suicidal ideation was reported by 2% of patients treated with deutetrabenazine, compared to no patients on placebo; no suicide attempts and no completed suicides were reported. Depression was reported by 4% of patients treated with deutetrabenazine.
- When considering the use of deutetrabenazine, the risk of suicidality should be balanced against the need for treatment of chorea. All patients treated with deutetrabenazine should be observed for new or worsening depression or suicidality. If depression or suicidality does not resolve, consider discontinuing treatment with deutetrabenazine.
- Patients, their caregivers, and families should be informed of the risks of depression, worsening depression, and suicidality associated with deutetrabenazine, and should be instructed to report behaviors of concern promptly to the treating physician. Patients with Huntington’s disease who express suicidal ideation should be evaluated immediately.
Clinical Worsening and Adverse Events in Patients with Huntington’s Disease
- Huntington’s disease is a progressive disorder characterized by changes in mood, cognition, chorea, rigidity, and functional capacity over time. VMAT2 inhibitors, including deutetrabenazine, may cause a worsening in mood, cognition, rigidity, and functional capacity.
- Prescribers should periodically re-evaluate the need for deutetrabenazine in their patients by assessing the effect on chorea and possible adverse effects, including sedation/somnolence, depression and suicidality, parkinsonism, akathisia, restlessness, and cognitive decline. It may be difficult to distinguish between adverse reactions and progression of the underlying disease; decreasing the dose or stopping the drug may help the clinician to distinguish between the two possibilities. In some patients, the underlying chorea itself may improve over time, decreasing the need for deutetrabenazine.
QTc Prolongation
- Tetrabenazine, a closely related VMAT2 inhibitor, causes an increase (about 8 msec) in the corrected QT (QTc) interval.
- A clinically relevant QT prolongation may occur in some patients treated with deutetrabenazine who are CYP2D6 poor metabolizers or are co-administered a strong CYP2D6 inhibitor [see Clinical Pharmacology.
- For patients who are CYP2D6 poor metabolizers or are taking a strong CYP2D6 inhibitor, dose reduction may be necessary. The use of deutetrabenazine in combination with other drugs that are known to prolong QTc may result in clinically significant QT prolongations.
- For patients requiring deutetrabenazine doses greater than 24 mg per day who are using deutetrabenazine with other drugs known to prolong QTc, assess the QTc interval before and after increasing the dose of deutetrabenazine or other medications that are known to prolong QTc.
- Deutetrabenazine should also be avoided in patients with congenital long QT syndrome and in patients with a history of cardiac arrhythmias. Certain circumstances may increase the risk of the occurrence of torsade de pointes and/or sudden death in association with the use of drugs that prolong the QTc interval, including (1) bradycardia; (2) hypokalemia or hypomagnesemia; (3) concomitant use of other drugs that prolong the QTc interval; and (4) presence of congenital prolongation of the QT interval.
Neuroleptic Malignant Syndrome (NMS)
- A potentially fatal symptom complex sometimes referred to as Neuroleptic Malignant Syndrome (NMS) has been reported in association with drugs that reduce dopaminergic transmission. While NMS has not been observed in patients receiving deutetrabenazine, it has been observed in patients receiving tetrabenazine (a closely related VMAT2 inhibitor). Clinicians should be alerted to the signs and symptoms associated with NMS. Clinical manifestations of NMS are hyperpyrexia, muscle rigidity, altered mental status, and evidence of autonomic instability (irregular pulse or blood pressure, tachycardia, diaphoresis, and cardiac dysrhythmia). Additional signs may include elevated creatinine phosphokinase, myoglobinuria, rhabdomyolysis, and acute renal failure. The diagnosis of NMS can be complicated; other serious medical illness (e.g., pneumonia, systemic infection) and untreated or inadequately treated extrapyramidal disorders can present with similar signs and symptoms. Other important considerations in the differential diagnosis include central anticholinergic toxicity, heat stroke, drug fever, and primary central nervous system pathology.
- The management of NMS should include (1) immediate discontinuation of deutetrabenazine; (2) intensive symptomatic treatment and medical monitoring; and (3) treatment of any concomitant serious medical problems for which specific treatments are available. There is no general agreement about specific pharmacological treatment regimens for NMS.
- Recurrence of NMS has been reported with resumption of drug therapy. If treatment with deutetrabenazine is needed after recovery from NMS, patients should be monitored for signs of recurrence.
Akathisia, Agitation, and Restlessness
- Deutetrabenazine may increase the risk of akathisia, agitation, and restlessness in patients with Huntington’s disease and tardive dyskinesia.
- In a 12-week, double-blind, placebo-controlled trial in Huntington’s disease patients, akathisia, agitation, or restlessness was reported by 4% of patients treated with deutetrabenazine, compared to 2% of patients on placebo; in patients with tardive dyskinesia, 2% of patients treated with deutetrabenazine and 1% of patients on placebo experienced these events.
- Patients receiving deutetrabenazine should be monitored for signs and symptoms of restlessness and agitation, as these may be indicators of developing akathisia. If a patient develops akathisia during treatment with deutetrabenazine, the deutetrabenazine dose should be reduced; some patients may require discontinuation of therapy.
Parkinsonism in Patients with Huntington’s Disease
- Deutetrabenazine may cause parkinsonism in patients with Huntington’s disease.
- Because rigidity can develop as part of the underlying disease process in Huntington’s disease, it may be difficult to distinguish between this potential drug-induced adverse reaction and progression of the underlying disease process. Drug-induced parkinsonism has the potential to cause more functional disability than untreated chorea for some patients with Huntington’s disease. If a patient develops parkinsonism during treatment with deutetrabenazine, the deutetrabenazine dose should be reduced; some patients may require discontinuation of therapy.
Sedation and Somnolence
- Sedation is a common dose-limiting adverse reaction of deutetrabenazine. In a 12-week, double-blind, placebo-controlled trial examining patients with Huntington’s disease, 11% of deutetrabenazine-treated patients reported somnolence compared with 4% of patients on placebo and 9% of deutetrabenazine-treated patients reported fatigue compared with 4% of placebo-treated patients.
- Patients should not perform activities requiring mental alertness to maintain the safety of themselves or others, such as operating a motor vehicle or operating hazardous machinery, until they are on a maintenance dose of deutetrabenazine and know how the drug affects them.
Hyperprolactinemia
- Serum prolactin levels were not evaluated in the deutetrabenazine development program. Tetrabenazine, a closely related VMAT2 inhibitor, elevates serum prolactin concentrations in humans. Following administration of 25 mg of tetrabenazine to healthy volunteers, peak plasma prolactin levels increased 4- to 5-fold.
- Tissue culture experiments indicate that approximately one-third of human breast cancers are prolactin-dependent in vitro, a factor of potential importance if deutetrabenazine is being considered for a patient with previously detected breast cancer. Although amenorrhea, galactorrhea, gynecomastia, and impotence can be caused by elevated serum prolactin concentrations, the clinical significance of elevated serum prolactin concentrations for most patients is unknown.
- Chronic increase in serum prolactin levels (although not evaluated in the deutetrabenazine or tetrabenazine development programs) has been associated with low levels of estrogen and increased risk of osteoporosis. If there is a clinical suspicion of symptomatic hyperprolactinemia, appropriate laboratory testing should be done and consideration should be given to discontinuation of deutetrabenazine.
Binding to Melanin-Containing Tissues
- Since deutetrabenazine or its metabolites bind to melanin-containing tissues, it could accumulate in these tissues over time. This raises the possibility that deutetrabenazine may cause toxicity in these tissues after extended use. Neither ophthalmologic nor microscopic examination of the eye has been conducted in the chronic toxicity studies in a pigmented species such as dogs. Ophthalmologic monitoring in humans was inadequate to exclude the possibility of injury occurring after long-term exposure.
- The clinical relevance of deutetrabenazine’s binding to melanin-containing tissues is unknown. Although there are no specific recommendations for periodic ophthalmologic monitoring, prescribers should be aware of the possibility of long-term ophthalmologic effects.
Adverse Reactions
Clinical Trials Experience
- Because clinical trials are conducted under widely varying conditions, adverse reaction rates observed in the clinical trials of a drug cannot be directly compared to rates in the clinical trials of another drug and may not reflect the rates observed in practice.
Patients with Huntington’s Disease
- Study 1 was a randomized, 12-week, placebo-controlled study in patients with chorea associated with Huntington’s disease. A total of 45 patients received deutetrabenazine, and 45 patients received placebo. Patients ranged in age between 23 and 74 years (mean 54 years); 56% were male, and 92% were Caucasian. The most common adverse reactions occurring in greater than 8% of deutetrabenazine-treated patients were somnolence, diarrhea, dry mouth, and fatigue. Adverse reactions occurring in 4% or more of patients treated with deutetrabenazine, and with a greater incidence than in patients on placebo, are summarized in Table 2.

- One or more adverse reactions resulted in a reduction of the dose of study medication in 7% of patients in Study 1. The most common adverse reaction resulting in dose reduction in patients receiving deutetrabenazine was dizziness (4%).
- Agitation led to discontinuation in 2% of patients treated with deutetrabenazine in Study 1.
Patients with Tardive Dyskinesia
- The data described below reflect 410 tardive dyskinesia patients participating in clinical trials. Deutetrabenazine was studied primarily in two 12-week, placebo-controlled trials (fixed dose, dose escalation). The population was 18 to 80 years of age, and had tardive dyskinesia and had concurrent diagnoses of mood disorder (33%) or schizophrenia/schizoaffective disorder (63%). In these studies, deutetrabenazine was administered in doses ranging from 12-48 mg per day. All patients continued on previous stable regimens of antipsychotics; 71% and 14% respective atypical and typical antipsychotic medications at study entry.
- The most common adverse reactions occurring in greater than 3% of deutetrabenazine-treated patients and greater than placebo were nasopharyngitis and insomnia. The adverse reactions occurring in >2% or more patients treated with deutetrabenazine (12-48 mg per day) and greater than in placebo patients in two double-blind, placebo-controlled studies in patients with tardive dyskinesia (Study 1 and Study 2) are summarized in Table 3.

- One or more adverse reactions resulted in a reduction of the dose of study medication in 4% of deutetrabenazine-treated patients and in 2% of placebo-treated patients.
Postmarketing Experience
There is limited information regarding Deutetrabenazine Postmarketing Experience in the drug label.
Drug Interactions
- Strong CYP2D6 Inhibitors
- Drugs that Cause QTc Prolongation
- Reserpine
- Monoamine Oxidase Inhibitors (MAOIs)
- Neuroleptic Drugs
- Alcohol or Other Sedating Drugs
- Concomitant Tetrabenazine or Valbenazine
Strong CYP2D6 Inhibitors
- A reduction in deutetrabenazine dose may be necessary when adding a strong CYP2D6 inhibitor in patients maintained on a stable dose of deutetrabenazine. Concomitant use of strong CYP2D6 inhibitors (e.g., paroxetine, fluoxetine, quinidine, bupropion) has been shown to increase the systemic exposure to the active dihydro-metabolites of deutetrabenazine by approximately 3-fold. The daily dose of deutetrabenazine should not exceed 36 mg per day, and the maximum single dose of deutetrabenazine should not exceed 18 mg in patients taking strong CYP2D6 inhibitors.
Drugs that Cause QTc Prolongation
- Tetrabenazine, a closely related VMAT2 inhibitor, may cause an increase in the corrected QT (QTc) interval. Clinically relevant QT prolongation may also occur with deutetrabanazine.
- For patients requiring deutetrabenazine doses above 24 mg per day, who are using deutetrabenazine in combination with other drugs known to prolong QTc, assess the QTc interval before and after increasing the dose of deutetrabenazine or other medications that are known to prolong QTc. Drugs known to prolong QTc include antipsychotic medications (e.g., chlorpromazine, haloperidol, thioridazine, ziprasidone), antibiotics (e.g., moxifloxacin), Class 1A (e.g., quinidine, procainamide), and Class III (e.g., amiodarone, sotalol) antiarrhythmic medications.
Reserpine
- Reserpine binds irreversibly to VMAT2 and the duration of its effect is several days. Prescribers should wait for chorea or dyskinesia to reemerge before administering deutetrabenazine to help reduce the risk of overdosage and major depletion of serotonin and norepinephrine in the central nervous system. At least 20 days should elapse after stopping reserpine before starting deutetrabenazine. Deutetrabenazine and reserpine should not be used concomitantly.
Monoamine Oxidase Inhibitors (MAOIs)
- Deutetrabenazine is contraindicated in patients taking MAOIs. Deutetramenazine should not be used in combination with an MAOI, or within 14 days of discontinuing therapy with an MAOI.
Neuroleptic Drugs
- The risk of parkinsonism, NMS, and akathisia may be increased by concomitant use of deutetrabenazine and dopamine antagonists or antipsychotics.
Alcohol or Other Sedating Drugs
- Concomitant use of alcohol or other sedating drugs may have additive effects and worsen sedation and somnolence.
Concomitant Tetrabenazine or Valbenazine
- Deutetrabenazine is contraindicated in patients currently taking tetrabenazine or valbenazine. Deutetrabenazine may be initiated the day following discontinuation of tetrabenazine.
Use in Specific Populations
Pregnancy
Risk Summary
- There are no adequate data on the developmental risk associated with the use of deutetrabenazine in pregnant women. Administration of deutetrabenazine to rats during organogenesis produced no clear adverse effect on embryofetal development. However, administration of tetrabenazine to rats throughout pregnancy and lactation resulted in an increase in stillbirths and postnatal offspring mortality.
- In the U.S. general population, the estimated background risk of major birth defects and miscarriage in clinically recognized pregnancies is 2-4% and 15-20%, respectively. The background risk of major birth defects and miscarriage for the indicated population is unknown.
Data (Animal)
- Oral administration of deutetrabenazine (5, 10, or 30 mg/kg/day) or tetrabenazine (30 mg/kg/day) to pregnant rats during organogenesis had no clear effect on embryofetal development. The highest dose tested was 6 times the maximum recommended human dose of 48 mg/day, on a body surface area (mg/m2) basis.
- The effects of deutetrabenazine when administered during organogenesis to rabbits or during pregnancy and lactation to rats have not been assessed.
- Tetrabenazine had no effects on embryofetal development when administered to pregnant rabbits during the period of organogenesis at oral doses up to 60 mg/kg/day. When tetrabenazine was administered to female rats (doses of 5, 15, and 30 mg/kg/day) from the beginning of organogenesis through the lactation period, an increase in stillbirths and offspring postnatal mortality was observed at 15 and 30 mg/kg/day, and delayed pup maturation was observed at all doses.
Pregnancy Category (AUS):
There is no Australian Drug Evaluation Committee (ADEC) guidance on usage of Deutetrabenazine in women who are pregnant.
Labor and Delivery
There is no FDA guidance on use of Deutetrabenazine during labor and delivery.
Nursing Mothers
Risk Summary
- There are no data on the presence of deutetrabenazine or its metabolites in human milk, the effects on the breastfed infant, or the effects of the drug on milk production.
- The developmental and health benefits of breastfeeding should be considered along with the mother’s clinical need for deutetrabenazine and any potential adverse effects on the breastfed infant from deutetrabenazine or from the underlying maternal condition.
Pediatric Use
- Safety and effectiveness in pediatric patients have not been established.
Geriatic Use
- Clinical studies of deutetrabenazine did not include sufficient numbers of subjects aged 65 and over to determine whether they respond differently from younger subjects. Other reported clinical experience has not identified differences in responses between the elderly and younger patients. In general, dose selection for an elderly patient should be cautious, usually starting at the low end of the dosing range, reflecting the greater frequency of hepatic, renal, and cardiac dysfunction, and of concomitant disease or other drug therapy.
Gender
There is no FDA guidance on the use of Deutetrabenazine with respect to specific gender populations.
Race
There is no FDA guidance on the use of Deutetrabenazine with respect to specific racial populations.
Renal Impairment
There is no FDA guidance on the use of Deutetrabenazine in patients with renal impairment.
Hepatic Impairment
- The effect of hepatic impairment on the pharmacokinetics of deutetrabenazine and its primary metabolites has not been studied; however, in a clinical study conducted with tetrabenazine, a closely related VMAT2 inhibitor, there was a large increase in exposure to tetrabenazine and its active metabolites in patients with hepatic impairment. The clinical significance of this increased exposure has not been assessed, but because of concerns for a greater risk for serious adverse reactions, the use of deutetrabenazine in patients with hepatic impairment is contraindicated.
Females of Reproductive Potential and Males
There is no FDA guidance on the use of Deutetrabenazine in women of reproductive potentials and males.
Immunocompromised Patients
There is no FDA guidance on the use of Deutetrabenazine in patients who are immunocompromised.
Poor CYP2D6 Metabolizers
- Although the pharmacokinetics of deutetrabenazine and its metabolites have not been systematically evaluated in patients who do not express the drug metabolizing enzyme, it is likely that the exposure to α-HTBZ and β-HTBZ would be increased similarly to taking a strong CYP2D6 inhibitor (approximately 3-fold). In patients who are CYP2D6 poor metabolizers, the daily dose of deutetrabenazine should not exceed 36 mg (maximum single dose of 18 mg.
Administration and Monitoring
Administration
- Oral
Monitoring
- Need for continued treatment: Periodically by assessing effect of treatment on chorea and possible adverse effects, including sedation/somnolence, depression and suicidality, parkinsonism, akathisia, restlessness, and cognitive decline; it may be difficult to distinguish between adverse reactions and progression of underlying disease.
- Emergence or worsening: Depression, suicidality, or unusual changes in behavior.
- Signs and symptoms of neuroleptic malignant syndrome.
- Signs and symptoms of developing akathisia, including restlessness and agitation.
IV Compatibility
There is limited information regarding the compatibility of Deutetrabenazine and IV administrations.
Overdosage
- Overdoses ranging from 100 mg to 1 g have been reported in the literature with tetrabenazine, a closely related VMAT2 inhibitor. The following adverse reactions occurred with overdosing: acute dystonia, oculogyric crisis, nausea and vomiting, sweating, sedation, hypotension, confusion, diarrhea, hallucinations, rubor, and tremor.
- Treatment should consist of those general measures employed in the management of overdosage with any central nervous system-active drug. General supportive and symptomatic measures are recommended. Cardiac rhythm and vital signs should be monitored. In managing overdosage, the possibility of multiple drug involvement should always be considered. The physician should consider contacting a poison control center on the treatment of any overdose.
Pharmacology
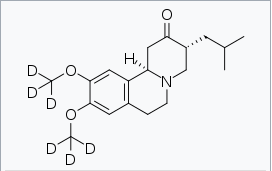
| |
Deutetrabenazine
| |
| Systematic (IUPAC) name | |
| (3R,11bR)-3-(2-Methylpropyl)-9,10-bis(trideuteriomethoxy)-1,3,4,6,7,11b-hexahydrobenzo[a]quinolizin-2-one | |
| Identifiers | |
| CAS number | |
| ATC code | ? |
| PubChem | |
| Chemical data | |
| Formula | Template:OrganicBox atomTemplate:OrganicBox atomTemplate:OrganicBoxTemplate:OrganicBoxTemplate:OrganicBoxTemplate:OrganicBoxTemplate:OrganicBoxTemplate:OrganicBoxTemplate:OrganicBoxTemplate:OrganicBoxTemplate:OrganicBoxTemplate:OrganicBoxTemplate:OrganicBoxTemplate:OrganicBox atomTemplate:OrganicBoxTemplate:OrganicBox atomTemplate:OrganicBoxTemplate:OrganicBoxTemplate:OrganicBoxTemplate:OrganicBoxTemplate:OrganicBoxTemplate:OrganicBoxTemplate:OrganicBox |
| Mol. mass | ? |
| Synonyms | Tetrabenazine D6; SD809; SD-809 |
| Pharmacokinetic data | |
| Bioavailability | ? |
| Metabolism | ? |
| Half life | ? |
| Excretion | ? |
| Therapeutic considerations | |
| Pregnancy cat. |
? |
| Legal status | |
| Routes | ? |
Mechanism of Action
- The precise mechanism by which deutetrabenazine exerts its effects in the treatment of tardive dyskinesia and chorea in patients with Huntington’s disease is unknown but is believed to be related to its effect as a reversible depletor of monoamines (such as dopamine, serotonin, norepinephrine, and histamine) from nerve terminals. The major circulating metabolites (α-dihydrotetrabenazine [HTBZ] and β-HTBZ) of deutetrabenazine, are reversible inhibitors of VMAT2, resulting in decreased uptake of monoamines into synaptic vesicles and depletion of monoamine stores.
Structure

Pharmacodynamics
Cardiac Electrophysiology
- The effect of a single 12-mg or 24-mg dose of deutetrabenazine on the QT interval was studied in a randomized, double-blind, placebo-controlled crossover study in healthy male and female subjects with moxifloxacin as a positive control. At 24 mg, deutetrabenazine caused an approximately 4.5 msec mean increase in QTc (90% CI: 2.4, 6.5 msec). Effects at higher exposures to deutetrabenazine or its metabolites have not been evaluated.
Melanin Binding
- Deutetrabenazine or its metabolites bind to melanin-containing tissues (i.e., eye, skin, fur) in pigmented rats. After a single oral dose of radiolabeled deutetrabenazine, radioactivity was still detected in eye and fur at 35 days following dosing.
Pharmacokinetics
- After oral dosing up to 25 mg, plasma concentrations of deutetrabenazine are generally below the limit of detection because of the extensive hepatic metabolism of deutetrabenazine to the active deuterated dihydro metabolites (HTBZ), α-HTBZ and β-HTBZ. Linear dose dependence of Cmax and AUC was observed for the active metabolites following single or multiple doses of deutetrabenazine (6 mg to 24 mg and 7.5 mg twice daily to 22.5 mg twice daily).
Absorption
- Following oral administration of deutetrabenazine, the extent of absorption is at least 80%.
- Plasma concentrations of deutetrabenazine are generally below the limit of detection after oral dosing. Peak plasma concentrations (Cmax) of deuterated α-HTBZ and β-HTBZ are reached within 3 to 4 hours after dosing.
Effect of Food
- The effects of food on the bioavailability of deutetrabenazine were studied in subjects administered a single dose with and without food. Food had no effect on the area under the plasma concentration-time curve (AUC) of α-HTBZ or β-HTBZ, although Cmax was increased by approximately 50% in the presence of food.
Distribution
- The median volume of distribution (Vc/F) of the α-HTBZ, and the β-HTBZ metabolites of deutetrabenazine are approximately 500 L and 730 L, respectively.
- Results of PET-scan studies in humans show that following intravenous injection of 11C-labeled tetrabenazine or α-HTBZ, radioactivity is rapidly distributed to the brain, with the highest binding in the striatum and lowest binding in the cortex.
- The in vitro protein binding of tetrabenazine, α-HTBZ, and β-HTBZ was examined in human plasma for concentrations ranging from 50 to 200 ng/mL. Tetrabenazine binding ranged from 82% to 85%, α-HTBZ binding ranged from 60% to 68%, and β-HTBZ binding ranged from 59% to 63%.
Elimination
- Deutetrabenazine is primarily renally eliminated in the form of metabolites.
- The half-life of total (α+β)-HTBZ from deutetrabenazine is approximately 9 to 10 hours.
- The median clearance values (CL/F) of the α-HTBZ, and the β-HTBZ metabolites of deutetrabenazine are approximately 47 L/hour and 70 L/hour, respectively, in the Huntington’s disease patient population.
Metabolism
- In vitro experiments in human liver microsomes demonstrate that deutetrabenazine is extensively biotransformed, mainly by carbonyl reductase, to its major active metabolites, α-HTBZ and β-HTBZ, which are subsequently metabolized primarily by CYP2D6, with minor contributions of CYP1A2 and CYP3A4/5, to form several minor metabolites.
Excretion
- In a mass balance study in 6 healthy subjects, 75% to 86% of the deutetrabenazine dose was excreted in the urine, and fecal recovery accounted for 8% to 11% of the dose. Urinary excretion of the α-HTBZ and β-HTBZ metabolites from deutetrabenazine each accounted for less than 10% of the administered dose. Sulfate and glucuronide conjugates of the α-HTBZ and β-HTBZ metabolites of deutetrabenazine, as well as products of oxidative metabolism, accounted for the majority of metabolites in the urine.
Specific Populations
Male and Female Patients
- There is no apparent effect of gender on the pharmacokinetics of α-HTBZ and β‑HTBZ of deutetrabenazine.
Patients with Renal Impairment
- No clinical studies have been conducted to assess the effect of renal impairment on the PK of deutetrabenazine.
Patients with Hepatic Impairment
- The effect of hepatic impairment on the pharmacokinetics of deutetrabenazine and its primary metabolites has not been studied. However, in a clinical study conducted to assess the effect of hepatic impairment on the pharmacokinetics of tetrabenazine, a closely related VMAT2 inhibitor, the exposure to α-HTBZ and β-HTBZ was up to 40% greater in patients with hepatic impairment, and the mean tetrabenazine Cmax in patients with hepatic impairment was up to 190-fold higher than in healthy subjects.
Poor CYP2D6 Metabolizers
- Although the pharmacokinetics of deutetrabenazine and its metabolites have not been systematically evaluated in patients who do not express the drug metabolizing enzyme CYP2D6, it is likely that the exposure to α-HTBZ and β-HTBZ would be increased similarly to taking strong CYP2D6 inhibitors (approximately 3-fold).
Drug Interaction Studies
- Deutetrabenazine, α-HTBZ, and β-HTBZ have not been evaluated in in vitro studies for induction or inhibition of CYP enzymes or interaction with P-glycoprotein. The results of in vitro studies of tetrabenazine do not suggest that tetrabenazine or its α-HTBZ or β-HTBZ metabolites are likely to result in clinically significant inhibition of CYP2D6, CYP1A2, CYP2B6, CYP2C8, CYP2C9, CYP2C19, CYP2E1, or CYP3A. In vitro studies suggest that neither tetrabenazine nor its α-HTBZ or β-HTBZ metabolites are likely to result in clinically significant induction of CYP1A2, CYP3A4, CYP2B6, CYP2C8, CYP2C9, or CYP2C19. Neither tetrabenazine nor its α-HTBZ or β-HTBZ metabolites are likely to be a substrate or inhibitor of P-glycoprotein at clinically relevant concentrations in vivo.
- The deutetrabenazine metabolites, 2-methylpropanoic acid of β-HTBZ (M1) and monohydroxy tetrabenazine (M4), have been evaluated in a panel of in vitro drug-drug interaction studies; the results indicate that M1/M4 are not expected to cause clinically relevant drug interactions.
CYP2D6 Inhibitors
- In vitro studies indicate that the α-HTBZ and β-HTBZ metabolites of deutetrabenazine are substrates for CYP2D6. The effect of CYP2D6 inhibition on the pharmacokinetics of deutetrabenazine and its metabolites was studied in 24 healthy subjects following a single 22.5 mg dose of deutetrabenazine given after 8 days of administration of the strong CYP2D6 inhibitor paroxetine 20 mg daily. In the presence of paroxetine, systemic exposure (AUCinf) of α-HTBZ was 1.9-fold higher and β-HTBZ was 6.5-fold higher, resulting in approximately 3-fold increase in AUCinf for total (α+β)-HTBZ. Paroxetine decreased the clearance of α-HTBZ and β-HTBZ metabolites of deutetrabenazine with corresponding increases in mean half-life of approximately 1.5-fold and 2.7-fold, respectively. In the presence of paroxetine, Cmax of α-HTBZ and β-HTBZ were 1.2-fold and 2.2-fold higher, respectively.
- The effect of moderate or weak CYP2D6 inhibitors such as duloxetine, terbinafine, amiodarone, or sertraline on the exposure of deutetrabenazine and its metabolites has not been evaluated.
Digoxin
- Deutetrabenazine was not evaluated for interaction with digoxin. Digoxin is a substrate for P-glycoprotein. A study in healthy subjects showed that tetrabenazine (25 mg twice daily for 3 days) did not affect the bioavailability of digoxin, suggesting that at this dose, tetrabenazine does not affect P‑glycoprotein in the intestinal tract. In vitro studies also do not suggest that tetrabenazine or its metabolites are P-glycoprotein inhibitors.
Nonclinical Toxicology
Carcinogenesis, Mutagenesis, Impairment of Fertility
Carcinogenesis
- No carcinogenicity studies were performed with deutetrabenazine.
- No increase in tumors was observed in p53+/– transgenic mice treated orally with tetrabenazine at doses of 0, 5, 15, and 30 mg/kg/day for 26 weeks.
Mutagenesis
- Deutetrabenazine and its deuterated α-HTBZ and β-HTBZ metabolites were negative in in vitro (bacterial reverse mutation and chromosome aberration in human peripheral blood lymphocytes) assays in the presence or absence of metabolic activation and in the in vivo micronucleus assay in mice.
Impairment of Fertility
- The effects of deutetrabenazine on fertility have not been evaluated. Oral administration of deutetrabenazine (doses of 5, 10, or 30 mg/kg/day) to female rats for 3 months resulted in estrous cycle disruption at all doses; the lowest dose tested was similar to the maximum recommended human dose (48 mg/day) on a body surface area (mg/m2) basis.
- Oral administration of tetrabenazine (doses of 5, 15, or 30 mg/kg/day) to female rats prior to and throughout mating, and continuing through day 7 of gestation, resulted in disrupted estrous cyclicity at doses greater than 5 mg/kg/day. No effects on mating and fertility indices or sperm parameters (motility, count, density) were observed when males were treated orally with tetrabenazine at doses of 5, 15 or 30 mg/kg/day prior to and throughout mating with untreated females.
Clinical Studies
Chorea Associated with Huntington’s Disease
- Double-Blind, Placebo-Controlled Study
- The efficacy of deutetrabenazine as a treatment for chorea associated with Huntington's disease was established primarily in Study 1, a randomized, double-blind, placebo-controlled, multi-center trial conducted in 90 ambulatory patients with manifest chorea associated with Huntington’s disease. The diagnosis of Huntington’s disease was based on family history, neurological exam, and genetic testing. Treatment duration was 12 weeks, including an 8-week dose titration period and a 4-week maintenance period, followed by a 1-week washout. Patients were not blinded to discontinuation. Deutetrabenazine was started at 6 mg per day and titrated upward, at weekly intervals, in 6 mg increments until satisfactory treatment of chorea was achieved, intolerable side effects occurred, or until a maximal dose of 48 mg per day was reached. The primary efficacy endpoint was the Total Maximal Chorea Score, an item of the Unified Huntington's Disease Rating Scale (UHDRS). On this scale, chorea is rated from 0 to 4 (with 0 representing no chorea) for 7 different parts of the body. The total score ranges from 0 to 28.
- Of the 90 patients enrolled, 87 patients completed the study. The mean age was 54 (range 23 to 74). Patients were 56% male and 92% Caucasian. The mean dose after titration was 40 mg per day. Table 4 and Figure 1 summarize the effects of deutetrabenazine on chorea based on the Total Maximal Chorea Score. Total Maximal Chorea Scores for patients receiving deutetrabenazine improved by approximately 4.4 units from baseline to the maintenance period (average of Week 9 and Week 12), compared to approximately 1.9 units in the placebo group. The treatment effect of -2.5 units was statistically significant (p<0.0001). The Maintenance Endpoint is the mean of the Total Maximal Chorea Scores for the Week 9 and Week 12 visits. At the Week 13 follow-up visit (1 week after discontinuation of the study medication), the Total Maximal Chorea Scores of patients who had received deutetrabenazine returned to baseline (Figure 1).
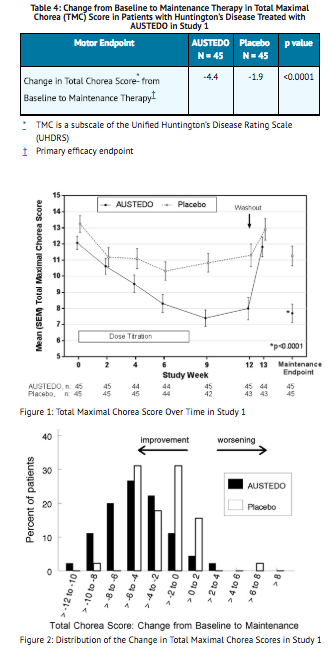
- Figure 2 shows the distribution of values for the change in Total Maximal Chorea Score in Study 1. Negative values indicate a reduction in chorea and positive numbers indicate an increase in chorea.
- A patient-rated global impression of change assessed how patients rated their overall Huntington’s disease symptoms. Fifty-one percent of patients treated with deutetrabenazine rated their symptoms as “Much Improved” or “Very Much Improved” at the end of treatment, compared to 20% of placebo-treated patients.
- In a physician-rated clinical global impression of change, 42% percent of patients treated with deutetrabenazine rated their symptoms as “Much Improved” or “Very Much Improved” at the end of treatment compared to 13% of placebo-treated patients.
Tardive Dyskinesia
- The efficacy of deutetrabenazine in the treatment for tardive dyskinesia was established in two 12‑week, randomized, double-blind, placebo-controlled, multi-center trials conducted in 335 adult ambulatory patients with tardive dyskinesia caused by use of dopamine receptor antagonists. Patients had a history of using a dopamine receptor antagonist (antipsychotics, metoclopramide) for at least 3 months (or 1 month in patients 60 years of age and older). Concurrent diagnoses included schizophrenia/schizoaffective disorder (62%) and mood disorder (33%). With respect to concurrent antipsychotic use, 64% of patients were receiving atypical antipsychotics, 12% were receiving typical or combination antipsychotics, and 24% were not receiving antipsychotics.
- The Abnormal Involuntary Movement Scale (AIMS) was the primary efficacy measure for the assessment of tardive dyskinesia severity. The AIMS is a 12-item scale; items 1 to 7 assess the severity of involuntary movements across body regions and these items were used in this study. Each of the 7 items was scored on a 0 to 4 scale, rated as: 0=not present; 1=minimal, may be extreme normal (abnormal movements occur infrequently and/or are difficult to detect); 2=mild (abnormal movements occur infrequently and are easy to detect); 3=moderate (abnormal movements occur frequently and are easy to detect) or 4 =severe (abnormal movements occur almost continuously and/or of extreme intensity). The AIMS total score (sum of items 1 to 7) could thus range from 0 to 28, with a decrease in score indicating improvement.
- In Study 1, a 12-week, placebo-controlled, fixed-dose trial, adults with tardive dyskinesia were randomized 1:1:1:1 to 12 mg deutetrabenazine, 24 mg deutetrabenazine, 36 mg deutetrabenazine, or placebo. Treatment duration included a 4-week dose escalation period and an 8-week maintenance period followed by a 1-week washout. The dose of deutetrabenazine was started at 12 mg per day and increased at weekly intervals in 6 mg/day increments to a dose target of 12 mg, 24 mg or 36 mg per day. The population (n= 222) was 21 to 81 years old (mean 57 years), 48% male, and 79% Caucasian. In Study 1, the AIMS total score for patients receiving deutetrabenazine demonstrated statistically significant improvement, from baseline to Week 12, of 3.3 and 3.2 units for the 36 mg and 24 mg arms, respectively, compared with 1.4 units in placebo (Study 1 in Table 5). The improvements on the AIMS total score over the course of the study are displayed in Figure 3. Data did not suggest substantial differences in efficacy across various demographic groups. The treatment response rate distribution, based on magnitude of AIMS total score from baseline to week 12 is displayed in Figure 4.
- The mean changes in the AIMS total score by visit are shown in Figure 3.
- In Study 2, a 12-week, placebo-controlled, flexible-dose trial, adults with tardive dyskinesia (n=113) received daily doses of placebo or deutetrabenazine, starting at 12 mg per day with increases allowed in 6-mg increments at 1-week intervals until satisfactory control of dyskinesia was achieved, until intolerable side effects occurred, or until a maximal dose of 48 mg per day was reached. Treatment duration included a 6-week dose titration period and a 6-week maintenance period followed by a 1-week washout. The population was 25 to 75 years old (mean 55 years), 48% male, and 70% Caucasian. Patients were titrated to an optimal dose over 6 weeks. The average dose of deutetrabenazine after treatment was 38.3 mg per day. There was no evidence suggesting substantial differences in efficacy across various demographic groups. In Study 2, AIMS total score for patients receiving deutetrabenazine demonstrated statistically significant improvement by 3.0 units from baseline to endpoint (Week 12), compared with 1.6 units in the placebo group with a treatment effect of -1.4 units. Table 5 summarizes the effects of deutetrabenazine on tardive dyskinesia based on the AIMS.



How Supplied
- Deutetrabenazine tablets are available in the following strengths and packages:
- 6 mg: round, purple-coated tablets, with “SD” over “6” printed in black ink on one side.
- Bottles of 60 tablets: NDC 68546-170-60.
- 9 mg: round, blue-coated tablets, with “SD” over “9” printed in black ink on one side.
- Bottles of 60 tablets: NDC 68546-171-60.
- 12 mg: round, beige-coated tablets, with “SD” over “12” printed in black ink on one side.
- Bottles of 60 tablets: NDC 68546-172-60.
Storage
- Store at 25ºC (77ºF); excursions permitted to 15ºC to 30ºC (59ºF to 86ºF). Protect from light and moisture.
Images
Drug Images
{{#ask: Page Name::Deutetrabenazine |?Pill Name |?Drug Name |?Pill Ingred |?Pill Imprint |?Pill Dosage |?Pill Color |?Pill Shape |?Pill Size (mm) |?Pill Scoring |?NDC |?Drug Author |format=template |template=DrugPageImages |mainlabel=- |sort=Pill Name }}
Package and Label Display Panel



{{#ask: Label Page::Deutetrabenazine |?Label Name |format=template |template=DrugLabelImages |mainlabel=- |sort=Label Page }}
Patient Counseling Information
- Advise the patient or caregiver to read the FDA-approved patient labeling.
Administration Instructions
- Advise patients to take deutetrabenazine with food. Deutetrabenazine tablets should be swallowed whole and not chewed, crushed, or broken.
Risk of Depression and Suicide in Patients with Huntington’s Disease
- Advise patients, their caregivers, and families that deutetrabenazine may increase the risk of depression, worsening depression, and suicidality, and to immediately report any symptoms to a healthcare provider.
Prolongation of the QTc Interval
- Inform patients to consult their physician immediately if they feel faint, lose consciousness, or have heart palpitations. Advise patients to inform physicians that they are taking deutetrabenazine before any new drug is taken.
Risk of Sedation and Somnolence
- Advise patients that deutetrabenazine may cause sedation and somnolence and may impair the ability to perform tasks that require complex motor and mental skills. Until they learn how they respond to a stable dose of deutetrabenazine, patients should be careful doing activities that require them to be alert, such as driving a car or operating machinery.
Interaction with Alcohol or Other Sedating Drugs
- Advise patients that alcohol or other drugs that cause sleepiness will worsen somnolence.
Concomitant Medications
- Advise patients to notify their physician of all medications they are taking and to consult with their healthcare provider before starting any new medications because of a potential for interactions.

Precautions with Alcohol
Alcohol-Deutetrabenazine interaction has not been established. Talk to your doctor regarding the effects of taking alcohol with this medication.
Brand Names
- Austedo
Look-Alike Drug Names
There is limited information regarding Deutetrabenazine Look-Alike Drug Names in the drug label.
Drug Shortage Status
Drug Shortage
Price
References
The contents of this FDA label are provided by the National Library of Medicine.