Denosumab
Editor-In-Chief: C. Michael Gibson, M.S., M.D. [1]; Associate Editor(s)-in-Chief: Sheng Shi, M.D. [2]; Sree Teja Yelamanchili, MBBS [3]
Disclaimer
WikiDoc MAKES NO GUARANTEE OF VALIDITY. WikiDoc is not a professional health care provider, nor is it a suitable replacement for a licensed healthcare provider. WikiDoc is intended to be an educational tool, not a tool for any form of healthcare delivery. The educational content on WikiDoc drug pages is based upon the FDA package insert, National Library of Medicine content and practice guidelines / consensus statements. WikiDoc does not promote the administration of any medication or device that is not consistent with its labeling. Please read our full disclaimer here.
Overview
Denosumab is a monoclonal antibody that is FDA approved for the treatment of postmenopausal women with osteoporosis at high risk for fracture, increase bone mass in men with osteoporosis, bone loss in men receiving androgen deprivation therapy for prostate cancer, bone loss in women receiving adjuvant aromatase inhibitor therapy for breast cancer. Common adverse reactions include hypercholesterolemia, diarrhea, nausea, vomiting, arthralgia, backache, pain in limb, asthenia, headache, cystitis, nasopharyngitis, upper respiratory infection, fatigue.
Adult Indications and Dosage
FDA-Labeled Indications and Dosage (Adult)
Recommended Dosage
- Denosumab should be administered by a healthcare professional.
- Recommended dose 60 mg SC injection once every 6 months.
- Administer Denosumab via subcutaneous injection in the upper arm, the upper thigh, or the abdomen.
- All patients should receive calcium 1000 mg daily and at least 400 IU vitamin D daily
- If a dose of Denosumab is missed, administer the injection as soon as the patient is available. Thereafter, schedule injections every 6 months from the date of the last injection.
Preparation and Administration
- Visually inspect Denosumab for particulate matter and discoloration prior to administration whenever solution and container permit. Denosumab is a clear, colorless to pale yellow solution that may contain trace amounts of translucent to white proteinaceous particles. Do not use if the solution is discolored or cloudy or if the solution contains many particles or foreign particulate matter.
- Latex Allergy: People sensitive to latex should not handle the grey needle cap on the single-use prefilled syringe, which contains dry natural rubber (a derivative of latex).
Prior to administration, Denosumab may be removed from the refrigerator and brought to room temperature (up to 25°C/77°F) by standing in the original container. This generally takes 15 to 30 minutes. Do not warm Denosumab in any other way.
Instructions for Prefilled Syringe with Needle Safety Guard
IMPORTANT: In order to minimize accidental needlesticks, the Denosumab single-use prefilled syringe will have a green safety guard; manually activate the safety guard after the injection is given. DO NOT slide the green safety guard forward over the needle before administering the injection; it will lock in place and prevent injection.

Activate the green safety guard (slide over the needle) after the injection. The grey needle cap on the single-use prefilled syringe contains dry natural rubber (a derivative of latex); people sensitive to latex should not handle the cap.
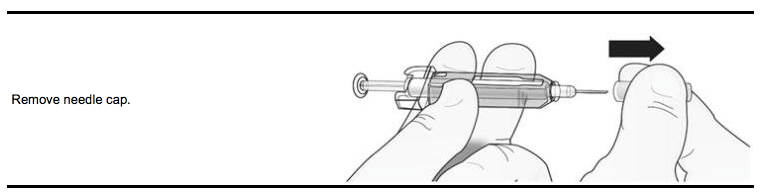
Step 1: Remove Grey Needle Cap
Step 2: Administer Subcutaneous Injection

DO NOT put grey needle cap back on needle.
Step 3: Immediately Slide Green Safety Guard Over Needle
With the needle pointing away from you… Hold the prefilled syringe by the clear plastic finger grip with one hand. Then, with the other hand, grasp the green safety guard by its base and gently slide it towards the needle until the green safety guard locks securely in place and/or you hear a “click.” DO NOT grip the green safety guard too firmly – it will move easily if you hold and slide it gently.

Immediately dispose of the syringe and needle cap in the nearest sharps container. DO NOT put the needle cap back on the used syringe.
Instructions for Single-use Vial
For administration of Denosumab from the single-use vial, use a 27-gauge needle to withdraw and inject the 1 mL dose. Do not re-enter the vial. Discard vial and any liquid remaining in the vial.
Off-Label Use and Dosage (Adult)
Guideline-Supported Use
There is limited information regarding Off-Label Guideline-Supported Use of Denosumab in adult patients.
Non–Guideline-Supported Use
There is limited information regarding Off-Label Non–Guideline-Supported Use of Denosumab in adult patients.
Pediatric Indications and Dosage
FDA-Labeled Indications and Dosage (Pediatric)
Denosumab is not recommended in pediatric patients
Off-Label Use and Dosage (Pediatric)
Guideline-Supported Use
There is limited information regarding Off-Label Guideline-Supported Use of Denosumab in pediatric patients.
Non–Guideline-Supported Use
There is limited information regarding Off-Label Non–Guideline-Supported Use of Denosumab in pediatric patients.
Contraindications
Hypocalcemia
Pre-existing hypocalcemia must be corrected prior to initiating therapy with Denosumab.
Pregnancy
Denosumab may cause fetal harm when administered to a pregnant woman. In utero denosumab exposure in cynomolgus monkeys resulted in increased fetal loss, stillbirths, and postnatal mortality, along with evidence of absent lymph nodes, abnormal bone growth and decreased neonatal growth. Denosumab is contraindicated in women who are pregnant. If this drug is used during pregnancy, or if the patient becomes pregnant while taking this drug, the patient should be apprised of the potential hazard to a fetus.
Hypersensitivity
Denosumab is contraindicated in patients with a history of systemic hypersensitivity to any component of the product. Reactions have included anaphylaxis, facial swelling and urticaria
Warnings
Drug Products with Same Active Ingredient
Denosumab contains the same active ingredient (denosumab) found in Xgeva. Patients receiving Denosumab should not receive Xgeva.
Hypersensitivity
Clinically significant hypersensitivity including anaphylaxis has been reported with Denosumab. Symptoms have included hypotension, dyspnea, throat tightness, facial and upper airway edema, pruritus, and urticaria. If an anaphylactic or other clinically significant allergic reaction occurs, initiate appropriate therapy and discontinue further use of Denosumab.
Hypocalcemia and Mineral Metabolism
Hypocalcemia may be exacerbated by the use of Denosumab. Pre-existing hypocalcemia must be corrected prior to initiating therapy with Denosumab. In patients predisposed to hypocalcemia and disturbances of mineral metabolism (e.g. history of hypoparathyroidism, thyroid surgery, parathyroid surgery, malabsorption syndromes, excision of small intestine, severe renal impairment (creatinine clearance <30 mL/min or receiving dialysis), clinical monitoring of calcium and mineral levels (phosphorus and magnesium) is highly recommended. Hypocalcemia following Denosumab administration is a significant risk in patients with severe renal impairment [creatinine clearance <30 mL/min] or receiving dialysis. These patients may also develop marked elevations of serum parathyroid hormone (PTH). Instruct all patients with severe renal impairment, including those receiving dialysis, about the symptoms of hypocalcemia and the importance of maintaining calcium levels with adequate calcium and vitamin D supplementation. Adequately supplement all patients with calcium and vitamin D.
Osteonecrosis of the Jaw
Osteonecrosis of the jaw (ONJ), which can occur spontaneously, is generally associated with tooth extraction and/or local infection with delayed healing. ONJ has been reported in patients receiving denosumab. A routine oral exam should be performed by the prescriber prior to initiation of Denosumab treatment. A dental examination with appropriate preventive dentistry should be considered prior to treatment with Denosumab in patients with risk factors for ONJ such as invasive dental procedures (e.g. tooth extraction, dental implants, oral surgery), diagnosis of cancer, concomitant therapies (e.g. chemotherapy, corticosteroids), poor oral hygiene, and co-morbid disorders (e.g. periodontal and/or other pre-existing dental disease, anemia, coagulopathy, infection, ill-fitting dentures). Good oral hygiene practices should be maintained during treatment with Denosumab. For patients requiring invasive dental procedures, clinical judgment of the treating physician and/or oral surgeon should guide the management plan of each patient based on individual benefit-risk assessment. Patients who are suspected of having or who develop ONJ while on Denosumab should receive care by a dentist or an oral surgeon. In these patients, extensive dental surgery to treat ONJ may exacerbate the condition. Discontinuation of Denosumab therapy should be considered based on individual benefit-risk assessment.
Atypical Subtrochanteric and Diaphyseal Femoral Fractures
Atypical low-energy or low trauma fractures of the shaft have been reported in patients receiving Denosumab. These fractures can occur anywhere in the femoral shaft from just below the lesser trochanter to above the supracondylar flare and are transverse or short oblique in orientation without evidence of comminution. Causality has not been established as these fractures also occur in osteoporotic patients who have not been treated with anti-resorptive agents. Atypical femoral fractures most commonly occur with minimal or no trauma to the affected area. They may be bilateral and many patients report prodromal pain in the affected area, usually presenting as dull, aching thigh pain, weeks to months before a complete fracture occurs. A number of reports note that patients were also receiving treatment with glucocorticoids (e.g. prednisone) at the time of fracture.During Denosumab treatment, patients should be advised to report new or unusual thigh, hip, or groin pain. Any patient who presents with thigh or groin pain should be suspected of having an atypical fracture and should be evaluated to rule out an incomplete femur fracture. Patient presenting with an atypical femur fracture should also be assessed for symptoms and signs of fracture in the contralateral limb. Interruption of Denosumab therapy should be considered, pending a risk/benefit assessment, on an individual basis.
Serious Infections
In a clinical trial of over 7800 women with postmenopausal osteoporosis, serious infections leading to hospitalization were reported more frequently in the Denosumab group than in the placebo group. Serious skin infections, as well as infections of the abdomen, urinary tract, and ear, were more frequent in patients treated with Denosumab. Endocarditis was also reported more frequently in Denosumab-treated patients. The incidence of opportunistic infections was similar between placebo and Denosumab groups, and the overall incidence of infections was similar between the treatment groups. Advise patients to seek prompt medical attention if they develop signs or symptoms of severe infection, including cellulitis. Patients on concomitant immunosuppressant agents or with impaired immune systems may be at increased risk for serious infections. Consider the benefit-risk profile in such patients before treating with Denosumab. In patients who develop serious infections while on Denosumab, prescribers should assess the need for continued Denosumab therapy.
Dermatologic Adverse Reactions
In a large clinical trial of over 7800 women with postmenopausal osteoporosis, epidermal and dermal adverse events such as dermatitis, eczema, and rashes occurred at a significantly higher rate in the Denosumab group compared to the placebo group. Most of these events were not specific to the injection site . Consider discontinuing Denosumab if severe symptoms develop.
Musculoskeletal Pain
In post-marketing experience, severe and occasionally incapacitating bone, joint, and/or muscle pain has been reported in patients taking Denosumab. The time to onset of symptoms varied from one day to several months after starting Denosumab. Consider discontinuing use if severe symptoms develop .
Suppression of Bone Turnover
In clinical trials in women with postmenopausal osteoporosis, treatment with Denosumab resulted in significant suppression of bone remodeling as evidenced by markers of bone turnover and bone histomorphometry. The significance of these findings and the effect of long-term treatment with Denosumab are unknown. The long-term consequences of the degree of suppression of bone remodeling observed with Denosumab may contribute to adverse outcomes such as osteonecrosis of the jaw, atypical fractures, and delayed fracture healing. Monitor patients for these consequences.
Adverse Reactions
Clinical Trials Experience
The following serious adverse reactions are discussed below and also elsewhere in the labeling:
- Hypocalcemia
- Serious Infections
- Dermatologic adverse reactions
- Osteonecrosis of the Jaw
- Atypical subtrochanteric and diaphyseal femoral fractures
The most common adverse reactions reported with Denosumab in patients with postmenopausal osteoporosis are back pain, pain in extremity, musculoskeletal pain, hypercholesterolemia, and cystitis. The most common adverse reactions reported with Denosumab in men with osteoporosis are back pain, arthralgia, and nasopharyngitis. The most common (per patient incidence ≥ 10%) adverse reactions reported with Denosumab in patients with bone loss receiving androgen deprivation therapy for prostate cancer or adjuvant aromatase inhibitor therapy for breast cancer are arthralgia and back pain. Pain in extremity and musculoskeletal pain have also been reported in clinical trials. The most common adverse reactions leading to discontinuation of Denosumab in patients with postmenopausal osteoporosis are back pain and constipation.
Because clinical studies are conducted under widely varying conditions, adverse reaction rates observed in the clinical studies of a drug cannot be directly compared to rates in the clinical studies of another drug and may not reflect the rates observed in clinical practice.
Treatment of Postmenopausal Women with Osteoporosis
The safety of Denosumab in the treatment of postmenopausal osteoporosis was assessed in a 3-year, randomized, double-blind, placebo-controlled, multinational study of 7808 postmenopausal women aged 60 to 91 years. A total of 3876 women were exposed to placebo and 3886 women were exposed to Denosumab administered subcutaneously once every 6 months as a single 60 mg dose. All women were instructed to take at least 1000 mg of calcium and 400 IU of vitamin D supplementation per day. The incidence of all-cause mortality was 2.3% (n = 90) in the placebo group and 1.8% (n = 70) in the Denosumab group. The incidence of nonfatal serious adverse events was 24.2% in the placebo group and 25.0% in the Denosumab group. The percentage of patients who withdrew from the study due to adverse events was 2.1% and 2.4% for the placebo and Denosumab groups, respectively. Adverse reactions reported in ≥ 2% of postmenopausal women with osteoporosis and more frequently in the Denosumab-treated women than in the placebo-treated women are shown in the table below.

Hypocalcemia
Decreases in serum calcium levels to less than 8.5 mg/dL at any visit were reported in 0.4% women in the placebo group and 1.7% women in the Denosumab group. The nadir in serum calcium level occurs at approximately day 10 after Denosumab dosing in subjects with normal renal function. In clinical studies, subjects with impaired renal function were more likely to have greater reductions in serum calcium levels compared to subjects with normal renal function. In a study of 55 subjects with varying degrees of renal function, serum calcium levels < 7.5 mg/dL or symptomatic hypocalcemia were observed in 5 subjects. These included no subjects in the normal renal function group, 10% of subjects in the creatinine clearance 50 to 80 mL/min group, 29% of subjects in the creatinine clearance < 30 mL/min group, and 29% of subjects in the hemodialysis group. These subjects did not receive calcium and vitamin D supplementation. In a study of 4550 postmenopausal women with osteoporosis, the mean change from baseline in serum calcium level 10 days after Denosumab dosing was -5.5% in subjects with creatinine clearance < 30 mL/min vs. -3.1% in subjects with creatinine clearance ≥ 30 mL/min.
Serious Infections
Receptor activator of nuclear factor kappa-B ligand (RANKL) is expressed on activated T and B lymphocytes and in lymph nodes. Therefore, a RANKL inhibitor such as Denosumab may increase the risk of infection. In the clinical study of 7808 postmenopausal women with osteoporosis, the incidence of infections resulting in death was 0.2% in both placebo and Denosumab treatment groups. However, the incidence of nonfatal serious infections was 3.3% in the placebo and 4.0% in the Denosumab groups. Hospitalizations due to serious infections in the abdomen (0.7% placebo vs. 0.9% Denosumab), urinary tract (0.5% placebo vs. 0.7% Denosumab), and ear (0.0% placebo vs. 0.1% Denosumab) were reported. Endocarditis was reported in no placebo patients and 3 patients receiving Denosumab. Skin infections, including erysipelas and cellulitis, leading to hospitalization were reported more frequently in patients treated with Denosumab (< 0.1% placebo vs. 0.4% Denosumab). The incidence of opportunistic infections was similar to that reported with placebo.
Dermatologic Reactions
A significantly higher number of patients treated with Denosumab developed epidermal and dermal adverse events (such as dermatitis, eczema, and rashes), with these events reported in 8.2% of the placebo and 10.8% of the Denosumab groups (p < 0.0001). Most of these events were not specific to the injection site.
Osteonecrosis of the Jaw
Osteonecrosis of the jaw has been reported in the osteoporosis clinical trial program in patients treated with Denosumab.
Atypical Subtrochanteric and Diaphyseal Fractures
In the osteoporosis clinical trial program, atypical femoral fractures were reported in patients treated with Denosumab. The duration of Denosumab exposure to time of atypical femoral fracture diagnosis was as early as 2½ years.
Pancreatitis
Pancreatitis was reported in 4 patients (0.1%) in the placebo and 8 patients (0.2%) in the Denosumab groups. Of these reports, 1 patient in the placebo group and all 8 patients in the Denosumab group had serious events, including one death in the Denosumab group. Several patients had a prior history of pancreatitis. The time from product administration to event occurrence was variable.
New Malignancies
The overall incidence of new malignancies was 4.3% in the placebo and 4.8% in the Denosumab groups. New malignancies related to the breast (0.7% placebo vs. 0.9% Denosumab), reproductive system (0.2% placebo vs. 0.5% Denosumab), and gastrointestinal system (0.6% placebo vs. 0.9% Denosumab) were reported. A causal relationship to drug exposure has not been established.
Treatment to Increase Bone Mass in Men with Osteoporosis
The safety of Denosumab in the treatment of men with osteoporosis was assessed in a 1-year randomized, double-blind, placebo-controlled study. A total of 120 men were exposed to placebo and 120 men were exposed to Denosumab administered subcutaneously once every 6 months as a single 60 mg dose. All men were instructed to take at least 1000 mg of calcium and 800 IU of vitamin D supplementation per day. The incidence of all-cause mortality was 0.8% (n = 1) in the placebo group and 0.8% (n = 1) in the Denosumab group. The incidence of nonfatal serious adverse events was 7.5% in the placebo group and 8.3% in the Denosumab group. The percentage of patients who withdrew from the study due to adverse events was 0% and 2.5% for the placebo and Denosumab groups, respectively. Adverse reactions reported in ≥ 5% of men with osteoporosis and more frequently with Denosumab than in the placebo-treated patients were: back pain (6.7% placebo vs. 8.3% Denosumab), arthralgia (5.8% placebo vs. 6.7% Denosumab), and nasopharyngitis (5.8% placebo vs. 6.7% Denosumab).
Serious Infections
Serious infection was reported in 1 patient (0.8%) in the placebo group and no patients in the Denosumab group.
Dermatologic Reactions
Epidermal and dermal adverse events (such as dermatitis, eczema, and rashes) were reported in 4 patients (3.3%) in the placebo group and 5 patients (4.2%) in the Denosumab group.
Osteonecrosis of the Jaw
No cases of ONJ were reported.
Pancreatitis
Pancreatitis was reported in 1 patient (0.8%) in the placebo group and 1 patient (0.8%) in the Denosumab group.
New Malignancies
New malignancies were reported in no patients in the placebo group and 4 (3.3%) patients (3 prostate cancers, 1 basal cell carcinoma) in the Denosumab group.
Treatment of Bone Loss in Patients Receiving Androgen Deprivation Therapy for Prostate Cancer or Adjuvant Aromatase Inhibitor Therapy for Breast Cancer
The safety of Denosumab in the treatment of bone loss in men with nonmetastatic prostate cancer receiving androgen deprivation therapy (ADT) was assessed in a 3‑year, randomized, double-blind, placebo-controlled, multinational study of 1468 men aged 48 to 97 years. A total of 725 men were exposed to placebo and 731 men were exposed to Denosumab administered once every 6 months as a single 60 mg subcutaneous dose. All men were instructed to take at least 1000 mg of calcium and 400 IU of vitamin D supplementation per day. The incidence of serious adverse events was 30.6% in the placebo group and 34.6% in the Denosumab group. The percentage of patients who withdrew from the study due to adverse events was 6.1% and 7.0% for the placebo and Denosumab groups, respectively. The safety of Denosumab in the treatment of bone loss in women with nonmetastatic breast cancer receiving aromatase inhibitor.
Postmarketing Experience
Because postmarketing reactions are reported voluntarily from a population of uncertain size, it is not always possible to reliably estimate their frequency or establish a causal relationship to drug exposure. The following adverse reactions have been identified during post approval use of Denosumab:
- Drug-related hypersensitivity reactions: anaphylaxis, rash, urticaria, facial swelling, and erythema
- Hypocalcemia: severe symptomatic hypocalcemia
- Musculoskeletal pain, including severe cases
- Parathyroid Hormone (PTH): Marked elevation in serum PTH in patients with severe renal impairment (creatinine clearance < 30 mL/min) or receiving dialysis.
Drug Interactions
In subjects with postmenopausal osteoporosis, Denosumab (60 mg subcutaneous injection) did not affect the pharmacokinetics of midazolam, which is metabolized by cytochrome P450 3A4 (CYP3A4), indicating that it should not affect the pharmacokinetics of drugs metabolized by this enzyme in this population.
Use in Specific Populations
Pregnancy
Pregnancy Category (FDA): X Risk Summary
Denosumab may cause fetal harm when administered to a pregnant woman based on findings in animals. In utero denosumab exposure in cynomolgus monkeys resulted in increased fetal loss, stillbirths, and postnatal mortality, along with evidence of absent lymph nodes, abnormal bone growth and decreased neonatal growth. Denosumab is contraindicated in women who are pregnant. If this drug is used during pregnancy, or if the patient becomes pregnant while taking this drug, the patient should be apprised of the potential hazard to a fetus. Women who become pregnant during Denosumab treatment are encouraged to enroll in Amgen’s Pregnancy Surveillance Program. Patients or their physicians should call 1-800-77-AMGEN (1-800-772-6436) to enroll.
Clinical Considerations
The effects of Denosumab on the fetus are likely to be greater during the second and third trimesters of pregnancy. Monoclonal antibodies, such as denosumab, are transported across the placenta in a linear fashion as pregnancy progresses, with the largest amount transferred during the third trimester. If the patient becomes pregnant during Denosumab therapy, treatment should be discontinued and the patient should consult their physician.
Animal Data
The effects of denosumab on prenatal development have been studied in both cynomolgus monkeys and genetically engineered mice in which RANK ligand (RANKL) expression was turned off by gene removal (a “knockout mouse”). In cynomolgus monkeys dosed subcutaneously with denosumab throughout pregnancy at a pharmacologically active dose, there was increased fetal loss during gestation, stillbirths, and postnatal mortality. Other findings in offspring included absence of axillary, inguinal, mandibular, and mesenteric lymph nodes; abnormal bone growth, reduced bone strength, reduced hematopoiesis, dental dysplasia and tooth malalignment; and decreased neonatal growth. At birth out to 1 month of age, infants had measurable blood levels of denosumab (22-621% of maternal levels).
Following a recovery period from birth out to 6 months of age, the effects on bone quality and strength returned to normal; there were no adverse effects on tooth eruption, though dental dysplasia was still apparent; axillary and inguinal lymph nodes remained absent, while mandibular and mesenteric lymph nodes were present, though small; and minimal to moderate mineralization in multiple tissues was seen in one recovery animal. There was no evidence of maternal harm prior to labor; adverse maternal effects occurred infrequently during labor. Maternal mammary gland development was normal. There was no fetal NOAEL (no observable adverse effect level) established for this study because only one dose of 50 mg/kg was evaluated.
In RANKL knockout mice, absence of RANKL (the target of denosumab) also caused fetal lymph node agenesis and led to postnatal impairment of dentition and bone growth. Pregnant RANKL knockout mice showed altered maturation of the maternal mammary gland, leading to impaired lactation
Pregnancy Category (AUS):
There is no Australian Drug Evaluation Committee (ADEC) guidance on usage of Denosumab in women who are pregnant.
Labor and Delivery
There is no FDA guidance on use of Denosumab during labor and delivery.
Nursing Mothers
It is not known whether Denosumab is excreted into human milk. Measurable concentrations of denosumab were present in the maternal milk of cynomolgus monkeys up to 1 month after the last dose of denosumab (≤ 0.5% milk:serum ratio). Because many drugs are excreted in human milk and because of the potential for serious adverse reactions in nursing infants from Denosumab, a decision should be made whether to discontinue nursing or discontinue the drug, taking into account the importance of the drug to the mother. Maternal exposure to Denosumab during pregnancy may impair mammary gland development and lactation based on animal studies in pregnant mice lacking the RANK/RANKL signaling pathway that have shown altered maturation of the maternal mammary gland, leading to impaired lactation postpartum. However in cynomolgus monkeys treated with denosumab throughout pregnancy, maternal mammary gland development was normal, with no impaired lactation. Mammary gland histopathology at 6 months of age was normal in female offspring exposed to denosumab in utero; however, development and lactation have not been fully evaluated
Pediatric Use
Denosumab is not recommended in pediatric patients. The safety and effectiveness of Denosumab in pediatric patients have not been established. Treatment with Denosumab may impair bone growth in children with open growth plates and may inhibit eruption of dentition. In neonatal rats, inhibition of RANKL (the target of Denosumab therapy) with a construct of osteoprotegerin bound to Fc (OPG-Fc) at doses ≤ 10 mg/kg was associated with inhibition of bone growth and tooth eruption. Adolescent primates treated with denosumab at doses 10 and 50 times (10 and 50 mg/kg dose) higher than the recommended human dose of 60 mg administered every 6 months, based on body weight (mg/kg), had abnormal growth plates, considered to be consistent with the pharmacological activity of denosumab. Cynomolgus monkeys exposed in utero to denosumab exhibited bone abnormalities, an absence of axillary, inguinal, mandibular, and mesenteric lymph nodes, reduced hematopoiesis, tooth malalignment, and decreased neonatal growth. Some bone abnormalities recovered once exposure was ceased following birth; however, axillary and inguinal lymph nodes remained absent 6 months post-birth.
Geriatic Use
Of the total number of patients in clinical studies of Denosumab, 9943 patients (76%) were ≥ 65 years old, while 3576 (27%) were ≥ 75 years old. Of the patients in the osteoporosis study in men, 133 patients (55%) were ≥ 65 years old, while 39 patients (16%) were ≥ 75 years old. No overall differences in safety or efficacy were observed between these patients and younger patients and other reported clinical experience has not identified differences in responses between the elderly and younger patients, but greater sensitivity of some older individuals cannot be ruled out.
Gender
Denosumab may cause fetal harm. The extent to which denosumab is present in seminal fluid is unknown. There is a potential for fetal exposure to denosumab when a man treated with Denosumab has unprotected sexual intercourse with a pregnant partner. The risk of fetal harm is likely to be low. Advise men being treated with Denosumab who have a pregnant partner of this potential risk.
Race
There is no FDA guidance on the use of Denosumab with respect to specific racial populations.
Renal Impairment
No dose adjustment is necessary in patients with renal impairment. In clinical studies, patients with severe renal impairment (creatinine clearance < 30 mL/min) or receiving dialysis were at greater risk of developing hypocalcemia. Consider the benefit-risk profile when administering Denosumab to patients with severe renal impairment or receiving dialysis. Clinical monitoring of calcium and mineral levels (phosphorus and magnesium) is highly recommended. Adequate intake of calcium and vitamin D is important in patients with severe renal impairment or receiving dialysis.
Hepatic Impairment
No clinical studies have been conducted to evaluate the effect of hepatic impairment on the pharmacokinetics of Denosumab.
Females of Reproductive Potential and Males
There is no FDA guidance on the use of Denosumab in women of reproductive potentials and males.
Immunocompromised Patients
There is no FDA guidance one the use of Denosumab in patients who are immunocompromised.
Administration and Monitoring
Administration
Subcutaneous injection
Monitoring
There is limited information about the drug monitoring.
IV Compatibility
There is limited information about the IV Compatibility.
Overdosage
There is no experience with overdosage with Denosumab.
Pharmacology
Denosumab?
| |
| Therapeutic monoclonal antibody | |
| Source | u |
| Target | RANK ligand |
| Identifiers | |
| CAS number | |
| ATC code | M05 |
| PubChem | ? |
| Chemical data | |
| Formula | Template:OrganicBox atomTemplate:OrganicBox atomTemplate:OrganicBoxTemplate:OrganicBoxTemplate:OrganicBoxTemplate:OrganicBoxTemplate:OrganicBoxTemplate:OrganicBoxTemplate:OrganicBoxTemplate:OrganicBoxTemplate:OrganicBoxTemplate:OrganicBoxTemplate:OrganicBoxTemplate:OrganicBox atomTemplate:OrganicBoxTemplate:OrganicBox atomTemplate:OrganicBoxTemplate:OrganicBoxTemplate:OrganicBox atomTemplate:OrganicBoxTemplate:OrganicBoxTemplate:OrganicBoxTemplate:OrganicBox |
| Mol. mass | 144.7 kDa |
| Synonyms | AMG 162 |
| Pharmacokinetic data | |
| Bioavailability | N/A |
| Metabolism | proteolysis |
| Half life | ? |
| Excretion | ? |
| Therapeutic considerations | |
| Licence data |
|
| Pregnancy cat. |
C(US) |
| Legal status |
[[Prescription drug|Template:Unicode-only]](US) |
| Routes | subcutaneous injection, every six months |
Mechanism of Action
Denosumab binds to RANKL, a transmembrane or soluble protein essential for the formation, function, and survival of osteoclasts, the cells responsible for bone resorption. Denosumab prevents RANKL from activating its receptor, RANK, on the surface of osteoclasts and their precursors. Prevention of the RANKL/RANK interaction inhibits osteoclast formation, function, and survival, thereby decreasing bone resorption and increasing bone mass and strength in both cortical and trabecular bone.
Structure
Denosumab (denosumab) is a human IgG2 monoclonal antibody with affinity and specificity for human RANKL (receptor activator of nuclear factor kappa-B ligand). Denosumab has an approximate molecular weight of 147 kDa and is produced in genetically engineered mammalian (Chinese hamster ovary) cells. Denosumab is a sterile, preservative-free, clear, colorless to pale yellow solution. Each 1 mL single-use prefilled syringe of Denosumab contains 60 mg denosumab (60 mg/mL solution), 4.7% sorbitol, 17 mM acetate, 0.01% polysorbate 20, Water for Injection (USP), and sodium hydroxide to a pH of 5.2. Each 1 mL single-use vial of Denosumab contains 60 mg denosumab (60 mg/mL solution), 4.7% sorbitol, 17 mM acetate, Water for Injection (USP), and sodium hydroxide to a pH of 5.2.
Pharmacodynamics
In clinical studies, treatment with 60 mg of Denosumab resulted in reduction in the bone resorption marker serum type 1 C-telopeptide (CTX) by approximately 85% by 3 days, with maximal reductions occurring by 1 month. CTX levels were below the limit of assay quantitation (0.049 ng/mL) in 39% to 68% of patients 1 to 3 months after dosing of Denosumab. At the end of each dosing interval, CTX reductions were partially attenuated from a maximal reduction of ≥ 87% to ≥ 45% (range: 45% to 80%), as serum denosumab levels diminished, reflecting the reversibility of the effects of Denosumab on bone remodeling. These effects were sustained with continued treatment. Upon reinitiation, the degree of inhibition of CTX by Denosumab was similar to that observed in patients initiating Denosumab treatment. Consistent with the physiological coupling of bone formation and resorption in skeletal remodeling, subsequent reductions in bone formation markers (i.e. osteocalcin and procollagen type 1 N-terminal peptide [PlNP]) were observed starting 1 month after the first dose of Denosumab. After discontinuation of Denosumab therapy, markers of bone resorption increased to levels 40% to 60% above pretreatment values but returned to baseline levels within 12 months.
Pharmacokinetics
In a study conducted in healthy male and female volunteers (n = 73, age range: 18 to 64 years) following a single subcutaneously administered Denosumab dose of 60 mg after fasting (at least for 12 hours), the mean maximum denosumab concentration (Cmax) was 6.75 mcg/mL (standard deviation [SD] = 1.89 mcg/mL). The median time to maximum denosumab concentration (Tmax) was 10 days (range: 3 to 21 days). After Cmax, serum denosumab concentrations declined over a period of 4 to 5 months with a mean half-life of 25.4 days (SD = 8.5 days; n = 46). The mean area-under-the-concentration-time curve up to 16 weeks (AUC0-16 weeks) of denosumab was 316 mcg×day/mL (SD = 101 mcg×day/mL). No accumulation or change in denosumab pharmacokinetics with time was observed upon multiple dosing of 60 mg subcutaneously administered once every 6 months. Denosumab pharmacokinetics were not affected by the formation of binding antibodies. A population pharmacokinetic analysis was performed to evaluate the effects of demographic characteristics. This analysis showed no notable differences in pharmacokinetics with age (in postmenopausal women), race, or body weight (36 to 140 kg).
Drug Interactions
In a study of 17 postmenopausal women with osteoporosis, midazolam (2 mg oral) was administered two weeks after a single dose of denosumab (60 mg subcutaneous injection), which approximates the Tmax of denosumab. Denosumab did not affect the pharmacokinetics of midazolam, which is metabolized by cytochrome P450 3A4 (CYP3A4). This indicates that denosumab should not alter the pharmacokinetics of drugs metabolized by CYP3A4 in postmenopausal women with osteoporosis.
Specific Populations
Gender:Mean serum denosumab concentration-time profiles observed in a study conducted in healthy men ≥ 50 years were similar to those observed in a study conducted in postmenopausal women using the same dose regimen. Age: The pharmacokinetics of denosumab were not affected by age across all populations studied whose ages ranged from 28 to 87 years. Race: The pharmacokinetics of denosumab were not affected by race. Renal Impairment: In a study of 55 patients with varying degrees of renal function, including patients on dialysis, the degree of renal impairment had no effect on the pharmacokinetics of denosumab; thus, dose adjustment for renal impairment is not necessary. Hepatic Impairment: No clinical studies have been conducted to evaluate the effect of hepatic impairment on the pharmacokinetics of denosumab.
Nonclinical Toxicology
Carcinogenesis, Mutagenesis, Impairment of Fertility
Carcinogenicity
The carcinogenic potential of denosumab has not been evaluated in long-term animal studies. Mutagenicity
The genotoxic potential of denosumab has not been evaluated.
Impairment of Fertility
Denosumab had no effect on female fertility or male reproductive organs in monkeys at doses that were 13- to 50-fold higher than the recommended human dose of 60 mg subcutaneously administered once every 6 months, based on body weight (mg/kg).
Animal Toxicology and/or Pharmacology
Denosumab is an inhibitor of osteoclastic bone resorption via inhibition of RANKL. In ovariectomized monkeys, once-monthly treatment with denosumab suppressed bone turnover and increased bone mineral density (BMD) and strength of cancellous and cortical bone at doses 50-fold higher than the recommended human dose of 60 mg administered once every 6 months, based on body weight (mg/kg). Bone tissue was normal with no evidence of mineralization defects, accumulation of osteoid, or woven bone. Because the biological activity of denosumab in animals is specific to nonhuman primates, evaluation of genetically engineered (“knockout”) mice or use of other biological inhibitors of the RANK/RANKL pathway, namely OPG-Fc, provided additional information on the pharmacodynamic properties of denosumab. RANK/RANKL knockout mice exhibited absence of lymph node formation, as well as an absence of lactation due to inhibition of mammary gland maturation (lobulo-alveolar gland development during pregnancy). Neonatal RANK/RANKL knockout mice exhibited reduced bone growth and lack of tooth eruption. A corroborative study in 2-week-old rats given the RANKL inhibitor OPG-Fc also showed reduced bone growth, altered growth plates, and impaired tooth eruption. These changes were partially reversible in this model when dosing with the RANKL inhibitors was discontinued.
Clinical Studies
Postmenopausal Women with Osteoporosis
The efficacy and safety of Denosumab in the treatment of postmenopausal osteoporosis was demonstrated in a 3-year, randomized, double-blind, placebo-controlled trial. Enrolled women had a baseline BMD T‑score between -2.5 and -4.0 at either the lumbar spine or total hip. Women with other diseases (such as rheumatoid arthritis, osteogenesis imperfecta, and Paget’s disease) or on therapies that affect bone were excluded from this study. The 7808 enrolled women were aged 60 to 91 years with a mean age of 72 years. Overall, the mean baseline lumbar spine BMD T-score was -2.8, and 23% of women had a vertebral fracture at baseline. Women were randomized to receive subcutaneous injections of either placebo (N = 3906) or Denosumab 60 mg (N = 3902) once every 6 months. All women received at least 1000 mg calcium and 400 IU vitamin D supplementation daily. The primary efficacy variable was the incidence of new morphometric (radiologically-diagnosed) vertebral fractures at 3 years. Vertebral fractures were diagnosed based on lateral spine radiographs (T4-L4) using a semiquantitative scoring method. Secondary efficacy variables included the incidence of hip fracture and nonvertebral fracture, assessed at 3 years.
Effect on Vertebral Fractures
Denosumab significantly reduced the incidence of new morphometric vertebral fractures at 1, 2, and 3 years (p < 0.0001), as shown in Table 2. The incidence of new vertebral fractures at year 3 was 7.2% in the placebo-treated women compared to 2.3% for the Denosumab-treated women. The absolute risk reduction was 4.8% and relative risk reduction was 68% for new morphometric vertebral fractures at year 3.

Denosumab was effective in reducing the risk for new morphometric vertebral fractures regardless of age, baseline rate of bone turnover, baseline BMD, baseline history of fracture, or prior use of a drug for osteoporosis.
Effect on Hip Fractures
The incidence of hip fracture was 1.2% for placebo-treated women compared to 0.7% for Denosumab-treated women at year 3. The age-adjusted absolute risk reduction of hip fractures was 0.3% with a relative risk reduction of 40% at 3 years (p = 0.04) (Figure 1).

Effect on Nonvertebral Fractures
Treatment with Denosumab resulted in a significant reduction in the incidence of nonvertebral fractures (Table 3)
.
Effect on Bone Mineral Density (BMD)
Treatment with Denosumab significantly increased BMD at all anatomic sites measured at 3 years. The treatment differences in BMD at 3 years were 8.8% at the lumbar spine, 6.4% at the total hip, and 5.2% at the femoral neck. Consistent effects on BMD were observed at the lumbar spine, regardless of baseline age, race, weight/body mass index (BMI), baseline BMD, and level of bone turnover. After Denosumab discontinuation, BMD returned to approximately baseline levels within 12 months.
Bone Histology and Histomorphometry
A total of 115 transiliac crest bone biopsy specimens were obtained from 92 postmenopausal women with osteoporosis at either month 24 and/or month 36 (53 specimens in Denosumab group, 62 specimens in placebo group). Of the biopsies obtained, 115 (100%) were adequate for qualitative histology and 7 (6%) were adequate for full quantitative histomorphometry assessment. Qualitative histology assessments showed normal architecture and quality with no evidence of mineralization defects, woven bone, or marrow fibrosis in patients treated with Denosumab. The presence of double tetracycline labeling in a biopsy specimen provides an indication of active bone remodeling, while the absence of tetracycline label suggests suppressed bone formation. In patients treated with Denosumab, 35% had no tetracycline label present at the month 24 biopsy and 38% had no tetracycline label present at the month 36 biopsy, while 100% of placebo-treated patients had double label present at both time points. When compared to placebo, treatment with Denosumab resulted in virtually absent activation frequency and markedly reduced bone formation rates. However, the long-term consequences of this degree of suppression of bone remodeling are unknown.
Treatment to Increase Bone Mass in Men with Osteoporosis
The efficacy and safety of Denosumab in the treatment to increase bone mass in men with osteoporosis was demonstrated in a 1-year, randomized, double-blind, placebo-controlled trial. Enrolled men had a baseline BMD T-score between -2.0 and -3.5 at the lumbar spine or femoral neck. Men with a BMD T-score between -1.0 and -3.5 at the lumbar spine or femoral neck were also enrolled if there was a history of prior fragility fracture. Men with other diseases (such as rheumatoid arthritis, osteogenesis imperfecta, and Paget’s disease) or on therapies that may affect bone were excluded from this study. The 242 men enrolled in the study ranged in age from 31 to 84 years with a mean age of 65 years. Men were randomized to receive SC injections of either placebo (n = 121) or Denosumab 60 mg (n = 121) once every 6 months. All men received at least 1000 mg calcium and at least 800 IU vitamin D supplementation daily.
Effect on Bone Mineral Density (BMD)
The primary efficacy variable was percent change in lumbar spine BMD from baseline to 1 year. Secondary efficacy variables included percent change in total hip, and femoral neck BMD from baseline to 1 year. Treatment with Denosumab significantly increased BMD at 1 year. The treatment differences in BMD at 1 year were 4.8% (+0.9% placebo, +5.7% Denosumab; (95% CI: 4.0, 5.6); p < 0.0001) at the lumbar spine, 2.0% (+0.3% placebo, +2.4% Denosumab) at the total hip, and 2.2% (0.0% placebo, +2.1% Denosumab) at femoral neck. Consistent effects on BMD were observed at the lumbar spine regardless of baseline age, race, BMD, testosterone concentrations and level of bone turnover.
Bone Histology and Histomorphometry
A total of 29 transiliac crest bone biopsy specimens were obtained from men with osteoporosis at 12 months (17 specimens in Denosumab group, 12 specimens in placebo group). Of the biopsies obtained, 29 (100%) were adequate for qualitative histology and, in Denosumab patients, 6 (35%) were adequate for full quantitative histomorphometry assessment. Qualitative histology assessments showed normal architecture and quality with no evidence of mineralization defects, woven bone, or marrow fibrosis in patients treated with Denosumab. The presence of double tetracycline labeling in a biopsy specimen provides an indication of active bone remodeling, while the absence of tetracycline label suggests suppressed bone formation. In patients treated with Denosumab, 6% had no tetracycline label present at the month 12 biopsy, while 100% of placebo-treated patients had double label present. When compared to placebo, treatment with Denosumab resulted in markedly reduced bone formation rates. However, the long-term consequences of this degree of suppression of bone remodeling are unknown.
Treatment of Bone Loss in Men with Prostate Cancer
The efficacy and safety of Denosumab in the treatment of bone loss in men with nonmetastatic prostate cancer receiving androgen deprivation therapy (ADT) were demonstrated in a 3‑year, randomized (1:1), double-blind, placebo-controlled, multinational study. Men less than 70 years of age had either a BMD T‑score at the lumbar spine, total hip, or femoral neck between ‑1.0 and -4.0, or a history of an osteoporotic fracture. The mean baseline lumbar spine BMD T-score was -0.4, and 22% of men had a vertebral fracture at baseline. The 1468 men enrolled ranged in age from 48 to 97 years (median 76 years). Men were randomized to receive subcutaneous injections of either placebo (n = 734) or Denosumab 60 mg (n = 734) once every 6 months for a total of 6 doses. Randomization was stratified by age (< 70 years vs. ≥ 70 years) and duration of ADT at trial entry (≤ 6 months vs. > 6 months). Seventy-nine percent of patients received ADT for more than 6 months at study entry. All men received at least 1000 mg calcium and 400 IU vitamin D supplementation daily.
Effect on Bone Mineral Density (BMD)
The primary efficacy variable was percent change in lumbar spine BMD from baseline to month 24. An additional key secondary efficacy variable was the incidence of new vertebral fracture through month 36 diagnosed based on x-ray evaluation by two independent radiologists. Lumbar spine BMD was higher at 2 years in Denosumab-treated patients as compared to placebo-treated patients [-1.0% placebo, +5.6% Denosumab; treatment difference 6.7% (95% CI: 6.2, 7.1); p < 0.0001]. With approximately 62% of patients followed for 3 years, treatment differences in BMD at 3 years were 7.9% (-1.2% placebo, +6.8% Denosumab) at the lumbar spine, 5.7% (-2.6% placebo, +3.2% Denosumab) at the total hip, and 4.9% (-1.8% placebo, +3.0% Denosumab) at the femoral neck. Consistent effects on BMD were observed at the lumbar spine in relevant subgroups defined by baseline age, BMD, and baseline history of vertebral fracture.
Effect on Vertebral Fractures
Denosumab significantly reduced the incidence of new vertebral fractures at 3 years (p = 0.0125), as shown in Table 4.

Treatment of Bone Loss in Women with Breast Cancer
The efficacy and safety of Denosumab in the treatment of bone loss in women receiving adjuvant aromatase inhibitor (AI) therapy for breast cancer was assessed in a 2‑year, randomized (1:1), double-blind, placebo-controlled, multinational study. Women had baseline BMD T-scores between ‑1.0 to ‑2.5 at the lumbar spine, total hip, or femoral neck, and had not experienced fracture after age 25. The mean baseline lumbar spine BMD T-score was -1.1, and 2.0% of women had a vertebral fracture at baseline. The 252 women enrolled ranged in age from 35 to 84 years (median 59 years). Women were randomized to receive subcutaneous injections of either placebo (n = 125) or Denosumab 60 mg (n = 127) once every 6 months for a total of 4 doses. Randomization was stratified by duration of adjuvant AI therapy at trial entry (≤ 6 months vs. > 6 months). Sixty-two percent of patients received adjuvant AI therapy for more than 6 months at study entry. All women received at least 1000 mg calcium and 400 IU vitamin D supplementation daily.
Effect on Bone Mineral Density (BMD)
The primary efficacy variable was percent change in lumbar spine BMD from baseline to month 12. Lumbar spine BMD was higher at 12 months in Denosumab-treated patients as compared to placebo-treated patients [-0.7% placebo, +4.8% Denosumab; treatment difference 5.5% (95% CI: 4.8, 6.3); p < 0.0001]. With approximately 81% of patients followed for 2 years, treatment differences in BMD at 2 years were 7.6% (-1.4% placebo, +6.2% Denosumab) at the lumbar spine, 4.7 % (-1.0% placebo, +3.8% Denosumab) at the total hip, and 3.6% (-0.8% placebo, +2.8% Denosumab) at the femoral neck.
How Supplied
Denosumab is supplied in a single-use prefilled syringe with a safety guard or in a single-use vial. The grey needle cap on the single-use prefilled syringe contains dry natural rubber (a derivative of latex).

Storage
Store Denosumab in a refrigerator at 2°C to 8°C (36°F to 46°F) in the original carton. Do not freeze. Prior to administration, Denosumab may be allowed to reach room temperature (up to 25°C/77°F) in the original container. Once removed from the refrigerator, Denosumab must not be exposed to temperatures above 25°C/77°F and must be used within 14 days. If not used within the 14 days, Denosumab should be discarded. Do not use Denosumab after the expiry date printed on the label. Protect Denosumab from direct light and heat. Avoid vigorous shaking of Denosumab.
Images
Drug Images
{{#ask: Page Name::Denosumab |?Pill Name |?Drug Name |?Pill Ingred |?Pill Imprint |?Pill Dosage |?Pill Color |?Pill Shape |?Pill Size (mm) |?Pill Scoring |?NDC |?Drug Author |format=template |template=DrugPageImages |mainlabel=- |sort=Pill Name }}
Package and Label Display Panel
{{#ask: Label Page::Denosumab |?Label Name |format=template |template=DrugLabelImages |mainlabel=- |sort=Label Page }}
Patient Counseling Information
See FDA-approved patient labeling (Medication Guide).
Drug Products with Same Active Ingredient
Advise patients that denosumab is also marketed as Xgeva, and if taking Denosumab, they should not receive Xgeva [see Warnings and Precautions (5.1)].
Hypersensitivity
Advise patients to seek prompt medical attention if signs or symptoms of hypersensitivity reactions occur. Advise patients who have had signs or symptoms of systemic hypersensitivity reactions that they should not receive denosumab (Denosumab or Xgeva) [see Warnings & Precautions (5.2), Contraindications (4.3)].
Hypocalcemia
Adequately supplement patients with calcium and vitamin D and instruct them on the importance of maintaining serum calcium levels while receiving Denosumab [see Warnings and Precautions (5.3) and Use in Specific Populations (8.6)]. Advise patients to seek prompt medical attention if they develop signs or symptoms of hypocalcemia.
Osteonecrosis of the Jaw
Advise patients to maintain good oral hygiene during treatment with Denosumab and to inform their dentist prior to dental procedures that they are receiving Denosumab. Patients should inform their physician or dentist if they experience persistent pain and/or slow healing of the mouth or jaw after dental surgery [see Warnings and Precautions (5.4)].
Atypical Subtrochanteric and Diaphyseal Femoral Fractures
Advise patients to report new or unusual thigh, hip, or groin pain [see Warnings and Precautions (5.5)].
Serious Infections
Advise patients to seek prompt medical attention if they develop signs or symptoms of infections, including cellulitis [see Warnings and Precautions (5.6)].
Dermatologic Reactions
Advise patients to seek prompt medical attention if they develop signs or symptoms of dermatological reactions (dermatitis, rashes, and eczema) [see Warnings and Precautions (5.7)].
Musculoskeletal Pain
Inform patients that severe bone, joint, and/or muscle pain have been reported in patients taking Denosumab. Patients should report severe symptoms if they develop [see Warnings and Precautions (5.8)].
Embryo-Fetal Toxicity
Pregnancy Advise patients that Denosumab is contraindicated in women who are pregnant and may cause fetal harm [see Contraindications (4.2), Use in Specific Populations (8.1)].
Males Advise patients of a potential for fetal exposure to denosumab when a man treated with Denosumab has unprotected sexual intercourse with a pregnant partner [see Use in Specific Populations (8.8)]
Nursing Mothers
Advise patients that because many drugs are excreted in human milk and because of the potential for serious adverse reactions in nursing infants from Denosumab, a decision should be made whether to discontinue nursing or discontinue the drug, taking into account the importance of the drug to the mother [see Use in Specific Populations (8.3)].
Schedule of Administration
If a dose of Denosumab is missed, administer the injection as soon as convenient. Thereafter, schedule injections every 6 months from the date of the last injection.
Precautions with Alcohol
Alcohol-Denosumab interaction has not been established. Talk to your doctor about the effects of taking alcohol with this medication.
Brand Names
- Prolia
- Xgeva
Look-Alike Drug Names
There is limited information about the look-alike names.
Drug Shortage Status
Price
References
The contents of this FDA label are provided by the National Library of Medicine.
{{#subobject:
|Label Page=Denosumab |Label Name=Denosumab_label_01.jpg
}}
{{#subobject:
|Label Page=Denosumab |Label Name=Denosumab_panel_01.png
}}