Delavirdine
Editor-In-Chief: C. Michael Gibson, M.S., M.D. [1]; Associate Editor(s)-in-Chief: Vignesh Ponnusamy, M.B.B.S. [2]
Disclaimer
WikiDoc MAKES NO GUARANTEE OF VALIDITY. WikiDoc is not a professional health care provider, nor is it a suitable replacement for a licensed healthcare provider. WikiDoc is intended to be an educational tool, not a tool for any form of healthcare delivery. The educational content on WikiDoc drug pages is based upon the FDA package insert, National Library of Medicine content and practice guidelines / consensus statements. WikiDoc does not promote the administration of any medication or device that is not consistent with its labeling. Please read our full disclaimer here.
Overview
Delavirdine is an antiretroviral agent that is FDA approved for the treatment of HIV infection. Common adverse reactions include rash, nausea, asthenia, headache, and fatigue.
Adult Indications and Dosage
FDA-Labeled Indications and Dosage (Adult)
HIV-1
- RESCRIPTOR Tablets are indicated for the treatment of HIV-1 infection in combination with at least 2 other active antiretroviral agents when therapy is warranted.
- The following should be considered before initiating therapy with RESCRIPTOR in treatment-naive patients. There are insufficient data directly comparing antiretroviral regimens containing RESCRIPTOR with currently preferred 3-drug regimens for initial treatment of HIV. In studies comparing regimens consisting of 2 nucleoside reverse transcriptase inhibitors (NRTIs) (currently considered suboptimal) to RESCRIPTOR plus 2 NRTIs, the proportion of patients receiving the regimen containing RESCRIPTOR who achieved and sustained an HIV-1 RNA level <400 copies/mL over 1 year of therapy was relatively low.
- Resistant virus emerges rapidly when RESCRIPTOR is administered as monotherapy. Therefore, RESCRIPTOR should always be administered in combination with other antiretroviral agents.
- The recommended dosage for RESCRIPTOR Tablets is 400 mg (four 100-mg or two 200-mg tablets) 3 times daily. RESCRIPTOR should be used in combination with other antiretroviral therapy. The complete prescribing information for other antiretroviral agents should be consulted for information on dosage and administration.
- The 100-mg RESCRIPTOR Tablets may be dispersed in water prior to consumption. To prepare a dispersion, add four 100-mg RESCRIPTOR Tablets to at least 3 ounces of water, allow to stand for a few minutes, and then stir until a uniform dispersion occurs. The dispersion should be consumed promptly. The glass should be rinsed with water and the rinse swallowed to insure the entire dose is consumed. The 200-mg tablets should be taken as intact tablets, because they are not readily dispersed in water. Note: The 200-mg tablets are approximately one-third smaller in size than the 100-mg tablets.
- RESCRIPTOR Tablets may be administered with or without food. Patients with achlorhydria should take RESCRIPTOR with an acidic beverage (e.g., orange or cranberry juice). However, the effect of an acidic beverage on the absorption of delavirdine in patients with achlorhydria has not been investigated.
- Patients taking both RESCRIPTOR and antacids should be advised to take them at least 1 hour apart.
Off-Label Use and Dosage (Adult)
Guideline-Supported Use
There is limited information regarding Off-Label Guideline-Supported Use of Delavirdine in adult patients.
Non–Guideline-Supported Use
There is limited information regarding Off-Label Non–Guideline-Supported Use of Delavirdine in adult patients.
Pediatric Indications and Dosage
FDA-Labeled Indications and Dosage (Pediatric)
- Safety and effectiveness of delavirdine in combination with other antiretroviral agents have not been established in HIV-1–infected individuals younger than 16 years of age.
Off-Label Use and Dosage (Pediatric)
Guideline-Supported Use
There is limited information regarding Off-Label Guideline-Supported Use of Delavirdine in pediatric patients.
Non–Guideline-Supported Use
There is limited information regarding Off-Label Non–Guideline-Supported Use of Delavirdine in pediatric patients.
Contraindications
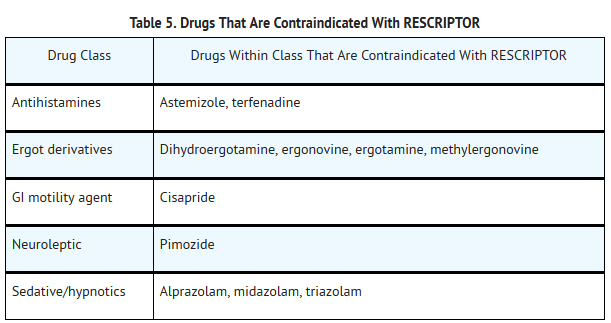
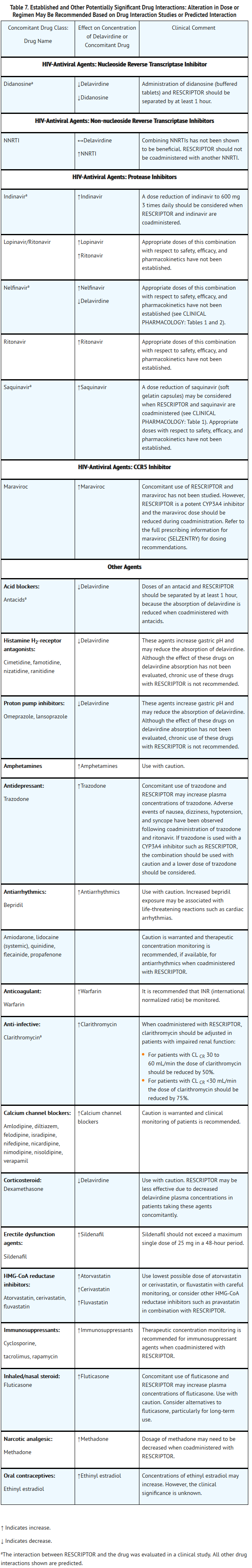
- RESCRIPTOR Tablets are contraindicated in patients with known hypersensitivity to any of its ingredients. Coadministration of RESCRIPTOR is contraindicated with drugs that are highly dependent on CYP3A for clearance and for which elevated plasma concentrations are associated with serious and/or life-threatening events. These drugs are listed in Table 5.
Warnings
- ALERT: Find out about medicines that should NOT be taken with RESCRIPTOR. This statement is included on the product’s bottle label.
- Drug Interactions
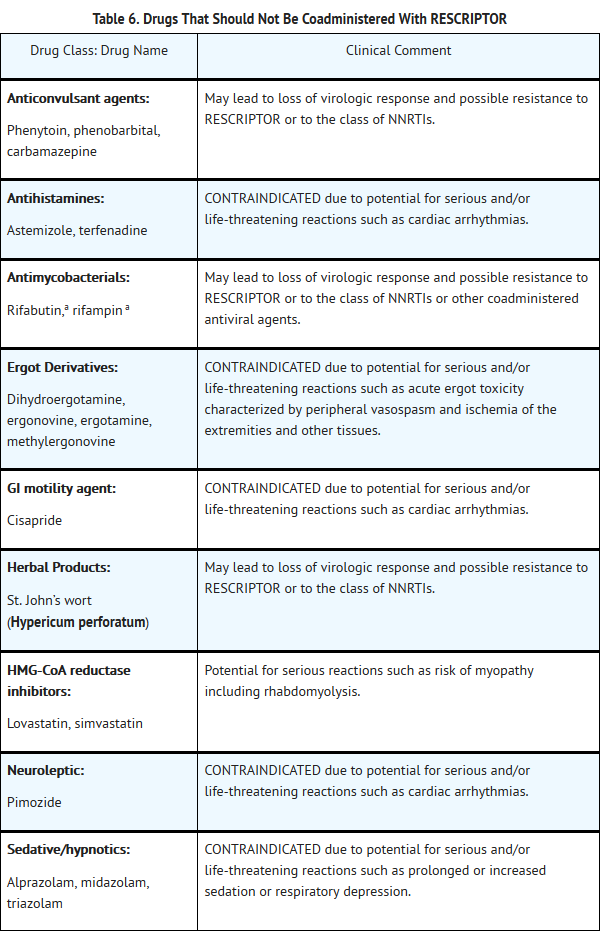
- Because delavirdine may inhibit the metabolism of many different drugs (e.g., antiarrhythmics, calcium channel blockers, sedative hypnotics, and others), serious and/or life-threatening drug interactions could result from inappropriate coadministration of some drugs with delavirdine. In addition, some drugs may markedly reduce delavirdine plasma concentrations, resulting in suboptimal antiviral activity and subsequent emergence of drug resistance. All prescribers should become familiar with the following tables in this package insert: Table 5, Drugs That Are Contraindicated With RESCRIPTOR;Table 6, Drugs That Should Not Be Coadministered With RESCRIPTOR;and Table 7, Established and Other Potentially Significant Drug Interactions: Alteration in Dose or Regimen May Be Recommended Based on Drug Interaction Studies or Predicted Interaction.
- Concomitant use of lovastatin or simvastatin with RESCRIPTOR is not recommended. Caution should be exercised if RESCRIPTOR is used concurrently with other HMG-CoA reductase inhibitors that are also metabolized by the CYP3A4 pathway (e.g., atorvastatin or cerivastatin). The risk of myopathy including rhabdomyolysis may be increased when RESCRIPTOR is used in combination with these drugs.
- Particular caution should be used when prescribing sildenafil in patients receiving RESCRIPTOR. Coadministration of sildenafil with RESCRIPTOR is expected to substantially increase sildenafil concentrations and may result in an increase in sildenafil-associated adverse events, including hypotension, visual changes, and priapism (see PRECAUTIONS: Drug Interactions and Information for Patients, and the complete prescribing information for sildenafil).
- Concomitant use of St. John’s wort (Hypericum perforatum) or St. John’s wort-containing products and RESCRIPTOR is not recommended. Coadministration of St. John’s wort with NNRTIs, including RESCRIPTOR, is expected to substantially decrease NNRTI concentrations and may result in suboptimal levels of RESCRIPTOR and lead to loss of virologic response and possible resistance to RESCRIPTOR or to the class of NNRTIs.
Precautions
- General
- Delavirdine is metabolized primarily by the liver. Therefore, caution should be exercised when administering RESCRIPTOR Tablets to patients with impaired hepatic function.
- Immune Reconstitution Syndrome
- Immune reconstitution syndrome has been reported in patients treated with combination antiretroviral therapy, including RESCRIPTOR. During the initial phase of the combination antiretroviral treatment, patients whose immune systems respond may develop an inflammatory response to indolent or residual opportunistic infections (such as Mycobacterium avium infection, cytomegalovirus, Pneumocystis jirovecii pneumonia [PCP], or tuberculosis), which may necessitate further evaluation and treatment.
- Autoimmune disorders (such as Graves’ disease, polymyositis, and Guillain-Barré syndrome) have also been reported to occur in the setting of immune reconstitution; however, the time to onset is more variable, and can occur many months after initiation of treatment.
- Resistance/Cross-Resistance
- NNRTIs, when used alone or in combination, may confer cross-resistance to other NNRTIs.
- Fat Redistribution
- Redistribution/accumulation of body fat including central obesity, dorsocervical fat enlargement (buffalo hump), peripheral wasting, facial wasting, breast enlargement, and "cushingoid appearance" have been observed in patients receiving antiretroviral therapy. The mechanism and long-term consequences of these events are currently unknown. A causal relationship has not been established.
- Skin Rash
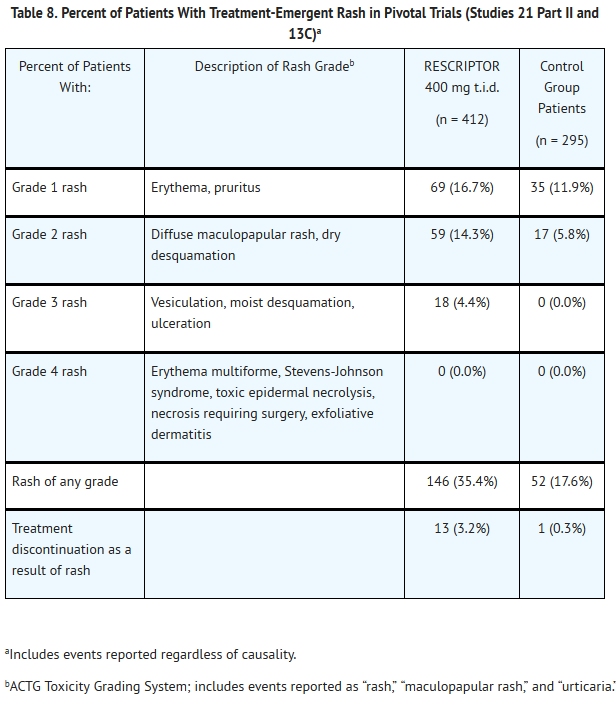
- Severe rash, including rare cases of erythema multiforme and Stevens-Johnson syndrome, has been reported in patients receiving RESCRIPTOR. Erythema multiforme and Stevens-Johnson syndrome were rarely seen in clinical trials and resolved after withdrawal of RESCRIPTOR. Any patient experiencing severe rash or rash accompanied by symptoms such as fever, blistering, oral lesions, conjunctivitis, swelling, and muscle or joint aches should discontinue RESCRIPTOR and consult a physician. Two cases of Stevens-Johnson syndrome have been reported through postmarketing surveillance out of a total of 339 surveillance reports.
- In Studies 21 Part II and 13C, rash (including maculopapular rash) was reported in more patients who were treated with RESCRIPTOR 400 mg 3 times daily (35% and 32%, respectively) than in those who were not treated with RESCRIPTOR (21% and 16%, respectively). The highest intensity of rash reported in these studies was severe (Grade 3), which was observed in approximately 4% of patients treated with RESCRIPTOR in each study and in none of the patients who were not treated with RESCRIPTOR. Also in Studies 21 Part II and 13C, discontinuations due to rash were reported in more patients who received RESCRIPTOR 400 mg 3 times daily (3% and 4%, respectively) than in those who did not receive RESCRIPTOR (0% and 1%, respectively).
- In most cases, the duration of the rash was less than 2 weeks and did not require dose reduction or discontinuation of RESCRIPTOR. Most patients were able to resume therapy after rechallenge with RESCRIPTOR following a treatment interruption due to rash. The distribution of the rash was mainly on the upper body and proximal arms, with decreasing intensity of the lesions on the neck and face, and progressively less on the rest of the trunk and limbs.
- Occurrence of a delavirdine-associated rash after 1 month is uncommon. Symptomatic relief has been obtained using diphenhydramine hydrochloride, hydroxyzine hydrochloride, and/or topical corticosteroids.
Adverse Reactions
Clinical Trials Experience
- The safety of RESCRIPTOR Tablets alone and in combination with other therapies has been studied in approximately 6,000 patients receiving RESCRIPTOR. The majority of adverse events were of mild or moderate (i.e., ACTG Grade 1 or 2) intensity. The most frequently reported drug-related adverse event (i.e., events considered by the investigator to be related to the blinded study medication or events with an unknown or missing causal relationship to the blinded medication) among patients receiving RESCRIPTOR was skin rash.
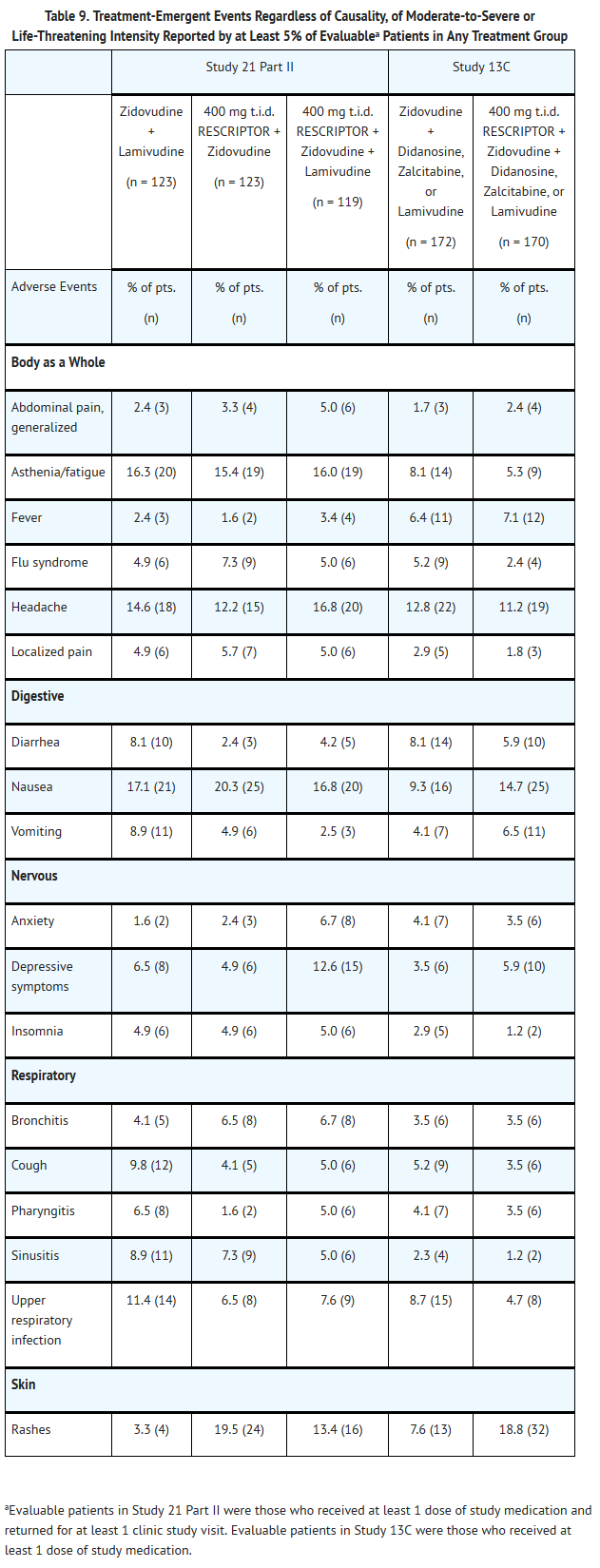
- Adverse events of moderate to severe intensity reported by at least 5% of evaluable patients in any treatment group in the pivotal trials, which includes patients receiving RESCRIPTOR in combination with zidovudine and/or lamivudine in Study 21 Part II for up to 98 weeks and in combination with zidovudine and either lamivudine, didanosine, or zalcitabine in Study 13C for up to 72 weeks are summarized in Table 9.
- Other Adverse Events in Phase II/III Studies
- Other adverse events that occurred in patients receiving RESCRIPTOR (in combination treatment) in all Phase II and III studies, considered possibly related to treatment, and of at least ACTG Grade 2 in intensity are listed below by body system.
Body as a Whole
Abdominal cramps, abdominal distention, abdominal pain (localized), abscess, allergic reaction, chills, edema (generalized or localized), epidermal cyst, fever, infection, infection viral, lip edema, malaise, Mycobacterium tuberculosis infection, neck rigidity, sebaceous cyst, and redistribution/accumulation of body fat.
Cardiovascular System
Abnormal cardiac rate and rhythm, cardiac insufficiency, cardiomyopathy, hypertension, migraine, pallor, peripheral vascular disorder, and postural hypotension.
Digestive System
Anorexia, bloody stool, colitis, constipation, decreased appetite, diarrhea (Clostridium difficile), diverticulitis, dry mouth, dyspepsia, dysphagia, enteritis at all levels, eructation, fecal incontinence, flatulence, gagging, gastroenteritis, gastroesophageal reflux, gastrointestinal bleeding, gastrointestinal disorder, gingivitis, gum hemorrhage, hepatomegaly, increased appetite, increased saliva, increased thirst, jaundice, mouth or tongue inflammation or ulcers, nonspecific hepatitis, oral/enteric moniliasis, pancreatitis, rectal disorder, sialadenitis, tooth abscess, and toothache.
Hemic and Lymphatic System
Adenopathy, bruising, eosinophilia, granulocytosis, leukopenia, pancytopenia, purpura, spleen disorder, thrombocytopenia, and prolonged prothrombin time.
Metabolic and Nutritional Disorders
Alcohol intolerance, amylase increased, bilirubinemia, hyperglycemia, hyperkalemia, hypertriglyceridemia, hyperuricemia, hypocalcemia, hyponatremia, hypophosphatemia, increased AST (SGOT), increased gamma glutamyl transpeptidase, increased lipase, increased serum alkaline phosphatase, increased serum creatinine, and weight increase or decrease.
Musculoskeletal System
Arthralgia or arthritis of single and multiple joints, bone disorder, bone pain, myalgia, tendon disorder, tenosynovitis, tetany, and vertigo.
Nervous System
Abnormal coordination, agitation, amnesia, change in dreams, cognitive impairment, confusion, decreased libido, disorientation, dizziness, emotional lability, euphoria, hallucination, hyperesthesia, hyperreflexia, hypertonia, hypesthesia, impaired concentration, manic symptoms, muscle cramp, nervousness, neuropathy, nystagmus, paralysis, paranoid symptoms, restlessness, sleep cycle disorder, somnolence, tingling, tremor, vertigo, and weakness.
Respiratory System
Chest congestion, dyspnea, epistaxis, hiccups, laryngismus, pneumonia, and rhinitis.
Skin and Appendages
Angioedema, dermal leukocytoclastic vasculitis, dermatitis, desquamation, diaphoresis, discolored skin, dry skin, erythema, erythema multiforme, folliculitis, fungal dermatitis, hair loss, herpes zoster or simplex, nail disorder, petechiae, non-application site pruritus, seborrhea, skin hypertrophy, skin disorder, skin nodule, Stevens-Johnson syndrome, urticaria, vesiculobullous rash, and wart.
Special Senses
Blepharitis, blurred vision, conjunctivitis, diplopia, dry eyes, ear pain, parosmia, otitis media, photophobia, taste perversion, and tinnitus.
Urogenital System
Amenorrhea, breast enlargement, calculi of the kidney, chromaturia, epididymitis, hematuria, hemospermia, impaired urination, impotence, kidney pain, metrorrhagia, nocturia, polyuria, proteinuria, testicular pain, urinary tract infection, and vaginal moniliasis.
Postmarketing Experience
- Adverse event terms reported from postmarketing surveillance that were not reported in the Phase II and III trials are presented below.
Digestive System
Hemic and Lymphatic System
Musculoskeletal System
Urogenital System
Drug Interactions
- Delavirdine is an inhibitor of CYP3A isoform and other CYP isoforms to a lesser extent including CYP2C9, CYP2D6, and CYP2C19. Coadministration of RESCRIPTOR and drugs primarily metabolized by CYP3A (e.g., HMG-CoA reductase inhibitors and sildenafil) may result in increased plasma concentrations of the coadministered drug that could increase or prolong both its therapeutic or adverse effects.
- Delavirdine is metabolized primarily by CYP3A, but in vitro data suggest that delavirdine may also be metabolized by CYP2D6. Coadministration of RESCRIPTOR and drugs that induce CYP3A, such as rifampin, may decrease delavirdine plasma concentrations and reduce its therapeutic effect. Coadministration of RESCRIPTOR and drugs that inhibit CYP3A may increase delavirdine plasma concentrations.
Use in Specific Populations
Pregnancy
- Pregnancy Category C
- Delavirdine has been shown to be teratogenic in rats. Delavirdine caused ventricular septal defects in rats at doses of 50, 100, and 200 mg/kg/day when administered during the period of organogenesis. The lowest dose of delavirdine that caused malformations produced systemic exposures in pregnant rats equal to or lower than the expected human exposure to RESCRIPTOR (Cmin 15 μM) at the recommended dose. Exposure in rats approximately 5-fold higher than the expected human exposure resulted in marked maternal toxicity, embryotoxicity, fetal developmental delay, and reduced pup survival. Additionally, reduced pup survival on postpartum day 0 occurred at an exposure (mean Cmin) approximately equal to the expected human exposure. Delavirdine was excreted in the milk of lactating rats at a concentration 3 to 5 times that of rat plasma.
- Delavirdine at doses of 200 and 400 mg/kg/day administered during the period of organogenesis caused maternal toxicity, embryotoxicity, and abortions in rabbits. The lowest dose of delavirdine that resulted in these toxic effects produced systemic exposures in pregnant rabbits approximately 6-fold higher than the expected human exposure to RESCRIPTOR (Cmin 15 μM) at the recommended dose. The no-observed-adverse-effect dose in the pregnant rabbit was 100 mg/kg/day. Various malformations were observed at this dose, but the incidence of such malformations was not statistically significantly different from that observed in the control group. Systemic exposures in pregnant rabbits at a dose of 100 mg/kg/day were lower than those expected in humans at the recommended clinical dose. Malformations were not apparent at 200 and 400 mg/kg/day; however, only a limited number of fetuses were available for examination as a result of maternal and embryo death.
- No adequate and well-controlled studies in pregnant women have been conducted. RESCRIPTOR should be used during pregnancy only if the potential benefit justifies the potential risk to the fetus. Of 9 pregnancies reported in premarketing clinical studies and postmarketing experience, a total of 10 infants were born (including 1 set of twins). Eight of the infants were born healthy. One infant was born HIV-positive but was otherwise healthy and with no congenital abnormalities detected, and 1 infant was born prematurely (34 to 35 weeks) with a small muscular ventricular septal defect that spontaneously resolved. The patient received approximately 6 weeks of treatment with delavirdine and zidovudine early in the course of the pregnancy.
- Antiretroviral Pregnancy Registry: To monitor maternal-fetal outcomes of pregnant women exposed to RESCRIPTOR and other antiretroviral agents, an Antiretroviral Pregnancy Registry has been established. Physicians are encouraged to register patients by calling (800) 258-4263.
- Australian Drug Evaluation Committee (ADEC) Pregnancy Category
There is no Australian Drug Evaluation Committee (ADEC) guidance on usage of Delavirdine in women who are pregnant.
Labor and Delivery
There is no FDA guidance on use of Delavirdine during labor and delivery.
Nursing Mothers
- The Centers for Disease Control and Prevention recommend that HIV-infected mothers not breastfeed their infants to avoid risking postnatal transmission of HIV. Because of both the potential for HIV transmission and any possible adverse reactions in nursing infants, mothers should be instructed not to breastfeed if they are receiving RESCRIPTOR.
Pediatric Use
- Safety and effectiveness of delavirdine in combination with other antiretroviral agents have not been established in HIV-1–infected individuals younger than 16 years of age.
Geriatic Use
- Clinical studies of RESCRIPTOR did not include sufficient numbers of subjects aged 65 and over to determine whether they respond differently from younger subjects. In general, caution should be taken when dosing RESCRIPTOR in elderly patients due to the greater frequency of decreased hepatic, renal, or cardiac function and of concomitant disease or other drug therapy.
Gender
There is no FDA guidance on the use of Delavirdine with respect to specific gender populations.
Race
There is no FDA guidance on the use of Delavirdine with respect to specific racial populations.
Renal Impairment
There is no FDA guidance on the use of Delavirdine in patients with renal impairment.
Hepatic Impairment
There is no FDA guidance on the use of Delavirdine in patients with hepatic impairment.
Females of Reproductive Potential and Males
There is no FDA guidance on the use of Delavirdine in women of reproductive potentials and males.
Immunocompromised Patients
There is no FDA guidance one the use of Delavirdine in patients who are immunocompromised.
Administration and Monitoring
Administration
- Oral
Monitoring
There is limited information regarding Monitoring of Delavirdine in the drug label.
IV Compatibility
There is limited information regarding IV Compatibility of Delavirdine in the drug label.
Overdosage
Acute Overdose
- Human experience of acute overdose with RESCRIPTOR is limited.
- Treatment of overdosage with RESCRIPTOR should consist of general supportive measures, including monitoring of vital signs and observation of the patient’s clinical status. There is no specific antidote for overdosage with RESCRIPTOR. If indicated, elimination of unabsorbed drug should be achieved by emesis or gastric lavage. Since delavirdine is extensively metabolized by the liver and is highly protein-bound, dialysis is unlikely to result in significant removal of the drug.
Chronic Overdose
There is limited information regarding Chronic Overdose of Delavirdine in the drug label.
Pharmacology
| |
| |
Delavirdine
| |
| Systematic (IUPAC) name | |
| N-[2-({4-[3-(propan-2-ylamino)pyridin-2-yl]piperazin-1-yl}carbonyl)-1H-indol-5-yl]methanesulfonamide | |
| Identifiers | |
| CAS number | |
| ATC code | J05 |
| PubChem | |
| DrugBank | |
| Chemical data | |
| Formula | Template:OrganicBox atomTemplate:OrganicBox atomTemplate:OrganicBoxTemplate:OrganicBoxTemplate:OrganicBoxTemplate:OrganicBoxTemplate:OrganicBoxTemplate:OrganicBoxTemplate:OrganicBoxTemplate:OrganicBoxTemplate:OrganicBoxTemplate:OrganicBoxTemplate:OrganicBoxTemplate:OrganicBox atomTemplate:OrganicBoxTemplate:OrganicBox atomTemplate:OrganicBoxTemplate:OrganicBoxTemplate:OrganicBox atomTemplate:OrganicBoxTemplate:OrganicBoxTemplate:OrganicBoxTemplate:OrganicBox |
| Mol. mass | 456.562 g/mol |
| SMILES | & |
| Pharmacokinetic data | |
| Bioavailability | 85% |
| Protein binding | 98% |
| Metabolism | Hepatic (CYP3A4- and CYP2D6-mediated) |
| Half life | 5.8 hours |
| Excretion | Renal (51%) and fecal (44%) |
| Therapeutic considerations | |
| Pregnancy cat. | |
| Legal status |
[[Prescription drug|Template:Unicode-only]](US) |
| Routes | Oral |
Mechanism of Action
- Delavirdine is an NNRTI of HIV-1. Delavirdine binds directly to reverse transcriptase (RT) and blocks RNA-dependent and DNA-dependent DNA polymerase activities. Delavirdine does not compete with template:primer or deoxynucleoside triphosphates. HIV-2 RT and human cellular DNA polymerases α, γ, or δ are not inhibited by delavirdine. In addition, HIV-1 group O, a group of highly divergent strains that are uncommon in North America, may not be inhibited by delavirdine.
Structure
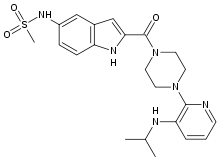
- RESCRIPTOR Tablets contain delavirdine mesylate, a synthetic non-nucleoside reverse transcriptase inhibitor (NNRTI) of the human immunodeficiency virus type 1 (HIV-1). The chemical name of delavirdine mesylate is piperazine, 1-[3-[(1-methyl-ethyl)amino]-2- pyridinyl]-4-[5-[(methylsulfonyl)amino]-1H-indol-2-yl]carbonyl]-, monomethanesulfonate. Its molecular formula is C22H28N6O3S•CH4O3S, and its molecular weight is 552.68. The structural formula is:delavirdine chemical structure
- Delavirdine mesylate is an odorless white-to-tan crystalline powder. The aqueous solubility of delavirdine free base at 23°C is 2,942 mcg/mL at pH 1.0, 295 mcg/mL at pH 2.0, and 0.81 mcg/mL at pH 7.4.
- Each RESCRIPTOR Tablet, for oral administration, contains 100 or 200 mg of delavirdine mesylate (henceforth referred to as delavirdine). Inactive ingredients consist of carnauba wax, colloidal silicon dioxide, croscarmellose sodium, lactose, magnesium stearate, and microcrystalline cellulose. In addition, the 100-mg tablet contains Opadry White YS-1-7000-E and the 200-mg tablet contains hypromellose and Opadry White YS-1-18202-A.
Pharmacodynamics
There is limited information regarding Pharmacodynamics of Delavirdine in the drug label.
Pharmacokinetics
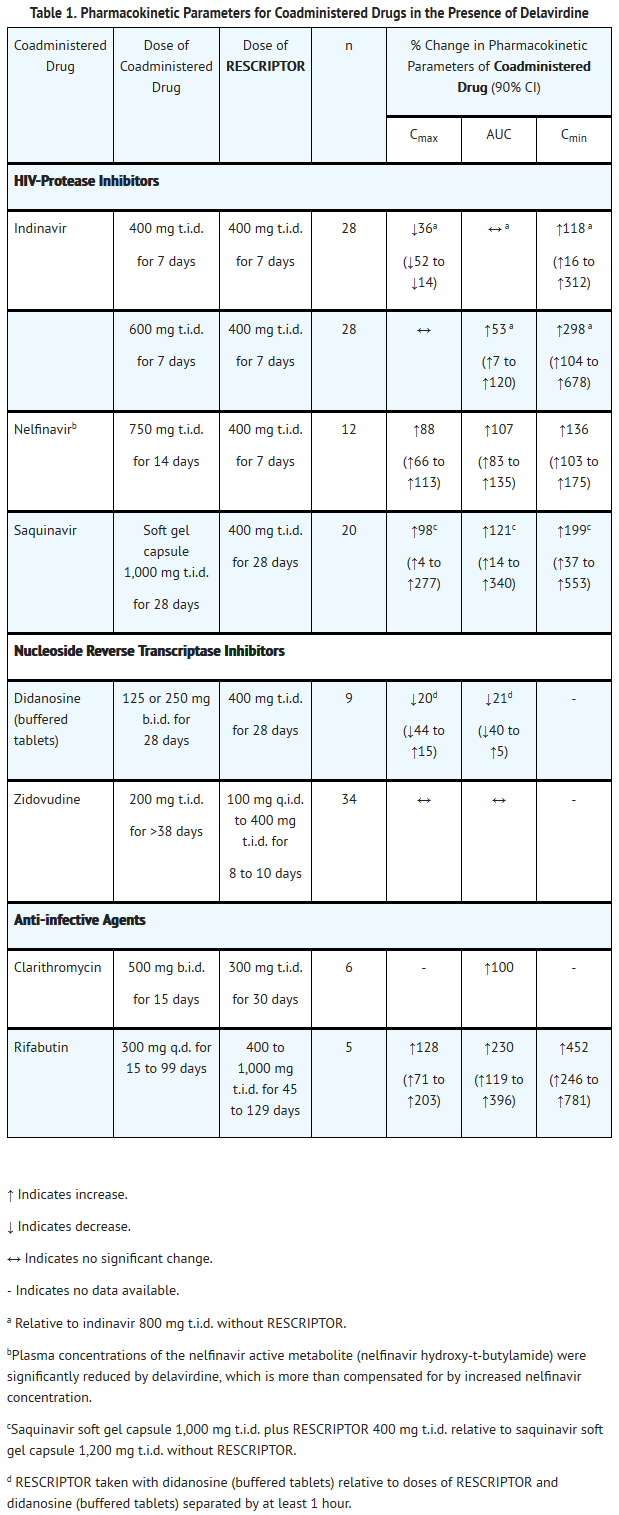
- Absorption and Bioavailability: Delavirdine is rapidly absorbed following oral administration, with peak plasma concentrations occurring at approximately 1 hour. Following administration of delavirdine 400 mg 3 times daily (n = 67, HIV-1–infected patients), the mean ±SD steady-state peak plasma concentration (Cmax) was 35 ± 20 μM (range: 2 to 100 μM), systemic exposure (AUC) was 180 ± 100 μM•hr (range: 5 to 515 μM•hr), and trough concentration (Cmin) was 15 ± 10 μM (range: 0.1 to 45 μM). The single-dose bioavailability of delavirdine tablets relative to an oral solution was 85% ± 25% (n = 16, non-HIV–infected subjects). The single-dose bioavailability of delavirdine tablets (100-mg strength) was increased by approximately 20% when a slurry of drug was prepared by allowing delavirdine tablets to disintegrate in water before administration (n = 16, non-HIV–infected subjects). The bioavailability of the 200-mg strength delavirdine tablets has not been evaluated when administered as a slurry because they are not readily dispersed in water.
- Delavirdine may be administered with or without food. In a multiple-dose, crossover study, delavirdine was administered every 8 hours with food or every 8 hours, 1 hour before or 2 hours after a meal (n = 13, HIV-1–infected patients). Patients remained on their typical diet throughout the study; meal content was not standardized. When multiple doses of delavirdine were administered with food, geometric mean Cmax was reduced by approximately 25%, but AUC and Cmin were not altered.
- Distribution: Delavirdine is extensively bound (approximately 98%) to plasma proteins, primarily albumin. The percentage of delavirdine that is protein-bound is constant over a delavirdine concentration range of 0.5 to 196 μM. In 5 HIV-1–infected patients whose total daily dose of delavirdine ranged from 600 to 1,200 mg, cerebrospinal fluid concentrations of delavirdine averaged 0.4% ± 0.07% of the corresponding plasma delavirdine concentrations; this represents about 20% of the fraction not bound to plasma proteins. Steady-state delavirdine concentrations in saliva (n = 5, HIV-1–infected patients who received delavirdine 400 mg 3 times daily) and semen (n = 5 healthy volunteers who received delavirdine 300 mg 3 times daily) were about 6% and 2%, respectively, of the corresponding plasma delavirdine concentrations collected at the end of a dosing interval.
- Metabolism and Elimination: Delavirdine is extensively converted to several inactive metabolites. Delavirdine is primarily metabolized by cytochrome P450 3A (CYP3A), but in vitro data suggest that delavirdine may also be metabolized by CYP2D6. The major metabolic pathways for delavirdine are N-desalkylation and pyridine hydroxylation. Delavirdine exhibits nonlinear steady-state elimination pharmacokinetics, with apparent oral clearance decreasing by about 22-fold as the total daily dose of delavirdine increases from 60 to 1,200 mg/day. In a study of 14C-delavirdine in 6 healthy volunteers who received multiple doses of delavirdine tablets 300 mg 3 times daily, approximately 44% of the radiolabeled dose was recovered in feces, and approximately 51% of the dose was excreted in urine. Less than 5% of the dose was recovered unchanged in urine. The parent plasma half-life of delavirdine increases with dose; mean half-life following 400 mg 3 times daily is 5.8 hours, with a range of 2 to 11 hours.
- In vitro and in vivo studies have shown that delavirdine reduces CYP3A activity and inhibits its own metabolism. In vitro studies have also shown that delavirdine reduces CYP2C9, CYP2D6, and CYP2C19 activity. Inhibition of hepatic CYP3A activity by delavirdine is reversible within 1 week after discontinuation of drug.
- Special Populations
- Hepatic or Renal Impairment: The pharmacokinetics of delavirdine in patients with hepatic or renal impairment have not been investigated.
- Age: The pharmacokinetics of delavirdine have not been adequately studied in patients aged <16 years or >65 years.
- Gender: Data from population pharmacokinetics suggest that the plasma concentrations of delavirdine tend to be higher in females than in males. However, this difference is not considered to be clinically significant.
- Race: No significant differences in the mean trough delavirdine concentrations were observed between different racial or ethnic groups.
- Drug Interactions
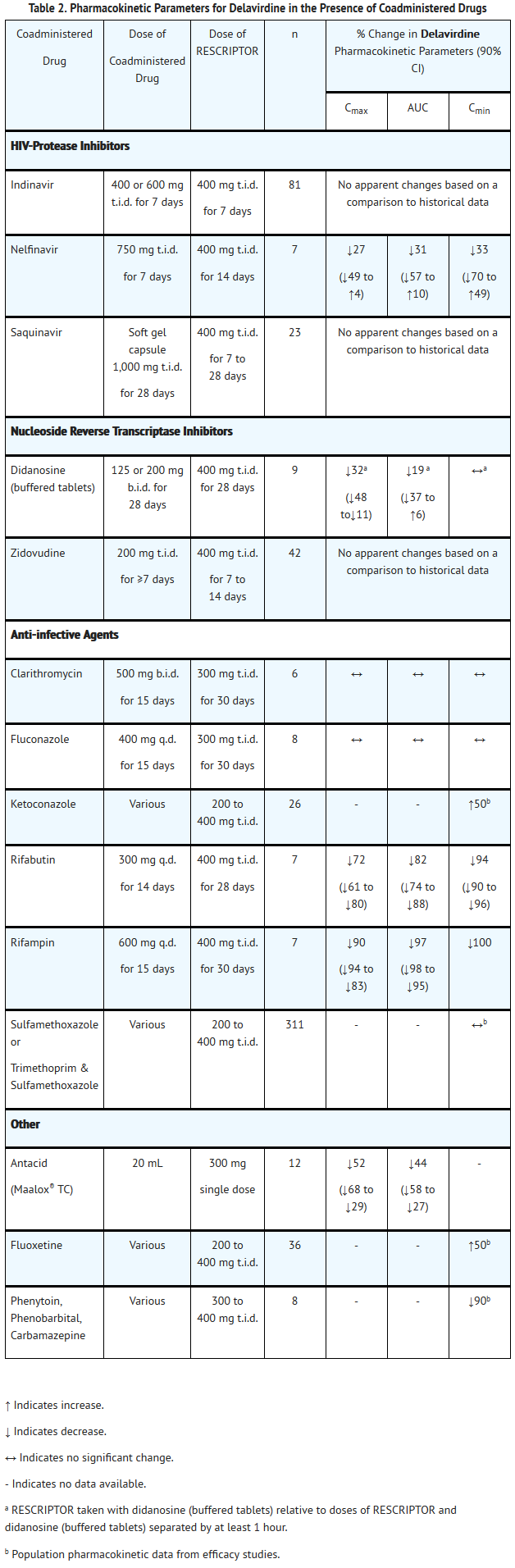
- Specific drug interaction studies were performed with delavirdine and a number of drugs. Table 1 summarizes the effects of delavirdine on the geometric mean AUC, Cmax, and Cmin of coadministered drugs. Table 2 shows the effects of coadministered drugs on the geometric mean AUC, Cmax, and Cmin of delavirdine.
Nonclinical Toxicology
- Delavirdine was negative in a battery of genetic toxicology tests which included an Ames assay, an in vitro rat hepatocyte unscheduled DNA synthesis assay, an in vitro chromosome aberration assay in human peripheral lymphocytes, an in vitro mutation assay in Chinese hamster ovary cells, and an in vivo micronucleus test in mice.
- Lifetime carcinogenicity studies were conducted in rats at doses of 10, 32, and 100 mg/kg/day and in mice at doses of 62.5, 250, and 500 mg/kg/day for males and 62.5, 125, and 250 mg/kg/day for females. In rats, delavirdine was noncarcinogenic at maximally tolerated doses that produced exposures (AUC) up to 12 (male rats) and 9 (female rats) times human exposure at the recommended clinical dose. In mice, delavirdine produced significant increases in the incidence of hepatocellular adenoma/adenocarcinoma in both males and females, hepatocellular adenoma in females, and mesenchymal urinary bladder tumors in males. The systemic drug exposures (AUC) in female mice were 0.5- to 3-fold and in male mice 0.2- to 4-fold of those in humans at the recommended clinical dose. Given the lack of genotoxic activity of delavirdine, the relevance of urinary bladder and hepatocellular neoplasm in delavirdine-treated mice to humans is not known.
- Delavirdine at doses of 20, 100, and 200 mg/kg/day did not cause impairment of fertility in rats when males were treated for 70 days and females were treated for 14 days prior to mating.
Clinical Studies
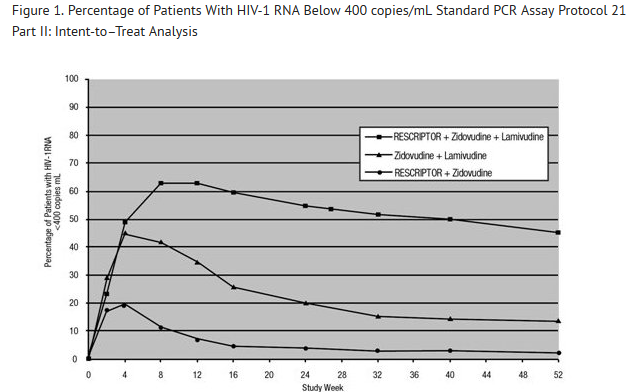
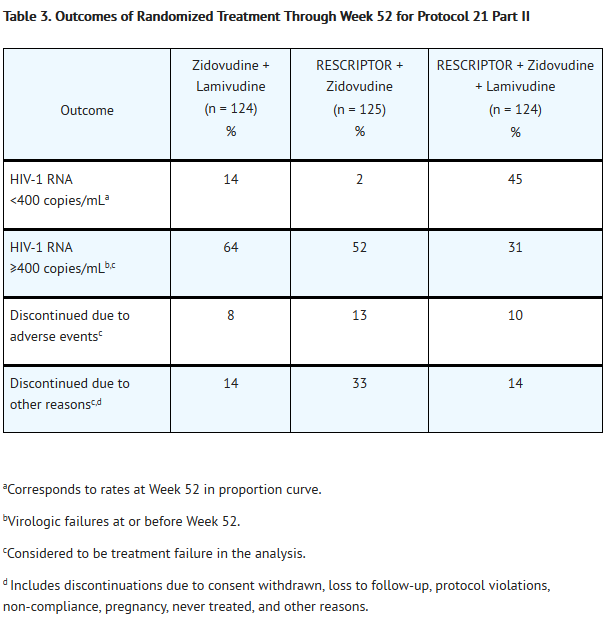
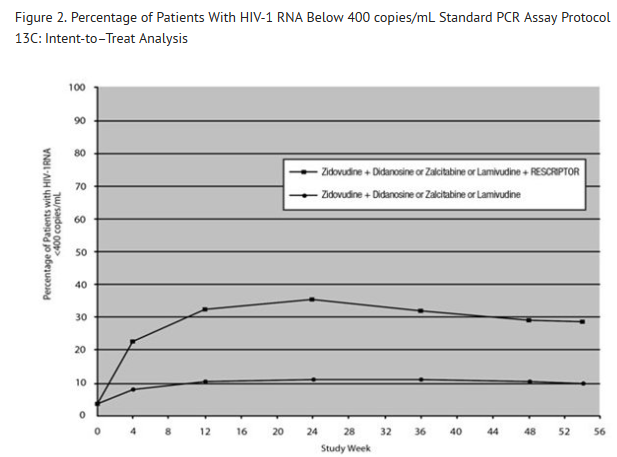
- For clinical Studies 21 Part II and 13C described below, efficacy was evaluated by the percentage of patients with a plasma HIV-1 RNA level <400 copies/mL through Week 52 as measured by the Roche Amplicor® HIV-1 Monitor (standard assay). An intent-to-treat analysis was performed where only subjects who achieved confirmed suppression and sustained it through Week 52 are regarded as responders. All other subjects (including never suppressed, discontinued, and those who rebounded after initial suppression of <400 copies/mL) are considered failures at Week 52. Results of an interim analysis of efficacy conducted for studies 21 Part II and 13C by independent Data and Safety Monitoring Boards (DSMBs) revealed that the triple-therapy arms in both studies produced significantly greater antiviral benefit than the dual-therapy arms, and early termination of the studies was recommended.
- Study 21 Part II
- Study 21 Part II was a double‑blind, randomized, placebo‑controlled trial comparing treatment with RESCRIPTOR (400 mg 3 times daily, zidovudine 200 mg 3 times daily, and lamivudine 150 mg twice daily versus RESCRIPTOR 400 mg 3 times daily and zidovudine 200 mg 3 times daily versus zidovudine 200 mg 3 times daily and lamivudine 150 mg twice daily in 373 HIV-1–infected patients (mean age 35 years [range: 17 to 67], 87% male, and 60% Caucasian) who were antiretroviral treatment naive (84%) or had limited nucleoside experience (16%). Mean baseline CD4+ cell count was 359 cells/mm3 and mean baseline plasma HIV‑1 RNA was 4.4 log10 copies/mL.
- Results showed that the mean increases from baseline in CD4 cell counts at 52 weeks were 111 cells/mL for RESCRIPTOR + zidovudine + lamivudine, 27 cells/mL for RESCRIPTOR + zidovudine, and 74 cells/mL for zidovudine + lamivudine.
- The results of the intent-to-treat analysis of the percentage of patients with a plasma HIV-1 RNA level <400 copies/mL are presented in Figure 1. HIV-1 RNA status and reasons for discontinuation of randomized treatment at 52 weeks are summarized in Table 3. Subjects who were never suppressed before discontinuation were placed in the discontinuation category.
- Figure 1. Percentage of Patients With HIV-1 RNA Below 400 copies/mL Standard PCR Assay Protocol 21 Part II: Intent-to–Treat Analysis
- Study 13C
- Study 13C was a double‑blind, randomized, placebo‑controlled trial comparing treatment with RESCRIPTOR 400 mg 3 times daily, zidovudine 200 mg 3 times daily or 300 mg twice daily, and either didanosine 200 mg twice daily, zalcitabine 0.75 mg 3 times daily, or lamivudine 150 mg twice daily versus zidovudine 200 mg 3 times daily or 300 mg twice daily and either didanosine 200 mg twice daily, zalcitabine 0.75 mg 3 times daily, or lamivudine 150 mg twice daily in 345 HIV-1–infected patients (mean age 35.8 years [range: 18 to 72], 66% male, and 63% Caucasian) who were antiretroviral treatment naive (63%) or had limited antiretroviral experience (37%). Mean baseline CD4+ cell count was 210 cells/mm3 and mean baseline plasma HIV‑1 RNA was 4.9 log10 copies/mL.
- Results showed that the mean increases from baseline in CD4+ cell counts at 54 weeks were 102 cells/mL for RESCRIPTOR + zidovudine + didanosine or zalcitabine or lamivudine, and 56 cells/mL for zidovudine + didanosine or zalcitabine or lamivudine.
- The results of the intent‑to‑treat analysis of the percentage of patients with a plasma HIV‑1 RNA level 400 copies/mL are presented in Figure 2. HIV‑1 RNA status and reasons for discontinuation of randomized treatment at 54 weeks are summarized in Table 4. Subjects who were never suppressed before discontinuation were placed in the discontinuation category.
- Figure 2. Percentage of Patients With HIV-1 RNA Below 400 copies/mL Standard PCR Assay Protocol 13C: Intent-to–Treat Analysis
How Supplied
- RESCRIPTOR Tablets are available as follows:
- 100-mg: white, capsule-shaped tablets marked with “U 3761”
- Bottles of 360 tablets - NDC 49702-209-24.
- 200-mg: white, capsule-shaped tablets marked with “RES200”
- Bottles of 180 tablets - NDC 49702-225-17.
- Store at controlled room temperature 20° to 25°C (68° to 77°F). Keep container tightly closed. Protect from high humidity.
Storage
There is limited information regarding Delavirdine Storage in the drug label.
Images
Drug Images
{{#ask: Page Name::Delavirdine |?Pill Name |?Drug Name |?Pill Ingred |?Pill Imprint |?Pill Dosage |?Pill Color |?Pill Shape |?Pill Size (mm) |?Pill Scoring |?NDC |?Drug Author |format=template |template=DrugPageImages |mainlabel=- |sort=Pill Name }}
Package and Label Display Panel
{{#ask: Label Page::Delavirdine |?Label Name |format=template |template=DrugLabelImages |mainlabel=- |sort=Label Page }}
Patient Counseling Information
- A statement to patients and healthcare providers is included on the product’s bottle label: ALERT: Find out about medicines that should NOT be taken with RESCRIPTOR. A patient package insert (PPI) for RESCRIPTOR is available for patient information.
- Patients should be informed that RESCRIPTOR is not a cure for HIV‑1 infection and that patients may continue to experience illnesses associated with HIV‑1 infection, including opportunistic infections. Patients should be advised to remain under the care of a physician while taking RESCRIPTOR.
- Patients should be advised to avoid doing things that can spread HIV-1 infection to others.
- Do not share needles or other injection equipment.
- Do not share personal items that can have blood or body fluids on them, like toothbrushes and razor blades.
- Do not have any kind of sex without protection. Always practice safe sex by using a latex or polyurethane condom to lower the chance of sexual contact with semen, vaginal secretions, or blood.
- Do not breastfeed. We do not know if RESCRIPTOR can be passed to your baby in your breast milk and whether it could harm your baby. Also, mothers with HIV-1 should not breastfeed because HIV-1 can be passed to the baby in the breast milk.
- Patients should be instructed that the major toxicity of RESCRIPTOR is rash and should be advised to promptly notify their physician should rash occur. The majority of rashes associated with RESCRIPTOR occur within 1 to 3 weeks after initiating treatment with RESCRIPTOR. The rash normally resolves in 3 to 14 days and may be treated symptomatically while therapy with RESCRIPTOR is continued. Any patient experiencing severe rash or rash accompanied by symptoms such as fever, blistering, oral lesions, conjunctivitis, swelling, and muscle or joint aches should discontinue medication and consult a physician.
- Patients should be informed that redistribution or accumulation of body fat may occur in patients receiving antiretroviral therapy and that the cause and long-term health effects of these conditions are not known at this time.
- Patients should be informed to take RESCRIPTOR every day as prescribed. Patients should not alter the dose of RESCRIPTOR without consulting their doctor. If a dose is missed, patients should take the next dose as soon as possible. However, if a dose is skipped, the patient should not double the next dose.
- Patients with achlorhydria should take RESCRIPTOR with an acidic beverage (e.g., orange or cranberry juice). However, the effect of an acidic beverage on the absorption of delavirdine in patients with achlorhydria has not been investigated.
- Patients taking both RESCRIPTOR and antacids should be advised to take them at least 1 hour apart.
- Because RESCRIPTOR may interact with certain drugs, patients should be advised to report to their doctor the use of any prescription, nonprescription medication, or herbal products, particularly St. John’s wort.
- Patients receiving sildenafil and RESCRIPTOR should be advised that they may be at an increased risk of sildenafil‑associated adverse events, including hypotension, visual changes, and prolonged penile erection, and should promptly report any symptoms to their doctor.
Precautions with Alcohol
- Alcohol-Delavirdine interaction has not been established. Talk to your doctor about the effects of taking alcohol with this medication.
Brand Names
- RESCRIPTOR®[1]
Look-Alike Drug Names
There is limited information regarding Delavirdine Look-Alike Drug Names in the drug label.
Drug Shortage Status
Price
References
The contents of this FDA label are provided by the National Library of Medicine.
{{#subobject:
|Page Name=Delavirdine
|Pill Name=No image.jpg
|Drug Name=
|Pill Ingred=|+sep=;
|Pill Imprint=
|Pill Dosage={{{dosageValue}}} {{{dosageUnit}}}
|Pill Color=|+sep=;
|Pill Shape=
|Pill Size (mm)=
|Pill Scoring=
|Pill Image=
|Drug Author=
|NDC=
}}
{{#subobject:
|Label Page=Delavirdine |Label Name=Delavirdine11.png
}}
{{#subobject:
|Label Page=Delavirdine |Label Name=Delavirdine12.png
}}
{{#subobject:
|Label Page=Delavirdine |Label Name=Delavirdine13.png
}}
{{#subobject:
|Label Page=Delavirdine |Label Name=Delavirdine14.png
}}