Deferiprone
Editor-In-Chief: C. Michael Gibson, M.S., M.D. [1]; Associate Editor(s)-in-Chief: Gloria Picoy [2]
Disclaimer
WikiDoc MAKES NO GUARANTEE OF VALIDITY. WikiDoc is not a professional health care provider, nor is it a suitable replacement for a licensed healthcare provider. WikiDoc is intended to be an educational tool, not a tool for any form of healthcare delivery. The educational content on WikiDoc drug pages is based upon the FDA package insert, National Library of Medicine content and practice guidelines / consensus statements. WikiDoc does not promote the administration of any medication or device that is not consistent with its labeling. Please read our full disclaimer here.
Black Box Warning
|
WARNING: AGRANULOCYTOSIS/NEUTROPENIA
See full prescribing information for complete Boxed Warning.
* Deferiprone can cause agranulocytosis that can lead to serious infections and death. Neutropenia may precede the development of agranulocytosis.
|
Overview
Deferiprone is a heavy metal chelator that is FDA approved for the treatment of patients with transfusional iron overload due to thalassemia syndromes when current chelation therapy is inadequate. There is a Black Box Warning for this drug as shown here. Common adverse reactions include urine discoloration, nausea, vomiting and abdominal pain, alanine aminotransferase increased, arthralgia and neutropenia.
Adult Indications and Dosage
FDA-Labeled Indications and Dosage (Adult)
- Deferiprone is indicated for the treatment of patients with transfusional iron overload due to thalassemia syndromes when current chelation therapy is inadequate.
- Initial dose is 25 mg/kg, orally, three times per day for a total of 75 mg/kg/day.
- The maximum dose is 33 mg/kg, three times per day for a total of 99 mg/kg/day.
Off-Label Use and Dosage (Adult)
Guideline-Supported Use
There is limited information regarding Off-Label Guideline-Supported Use of Deferiprone in adult patients.
Non–Guideline-Supported Use
There is limited information regarding Off-Label Non–Guideline-Supported Use of Deferiprone in adult patients.
Pediatric Indications and Dosage
FDA-Labeled Indications and Dosage (Pediatric)
Safety and efficacy has not been established in pediatric patients
Off-Label Use and Dosage (Pediatric)
Guideline-Supported Use
There is limited information regarding Off-Label Guideline-Supported Use of Deferiprone in pediatric patients.
Non–Guideline-Supported Use
There is limited information regarding Off-Label Non–Guideline-Supported Use of Deferiprone in pediatric patients.
Contraindications
Deferiprone is contraindicated in patients with known hypersensitivity to deferiprone or to any of the excipients in the formulation. The following reactions have been reported in association with the administration of deferiprone: Henoch-Schönlein purpura; urticaria; and periorbital edema with skin rash
Warnings
|
WARNING: AGRANULOCYTOSIS/NEUTROPENIA
See full prescribing information for complete Boxed Warning.
* Deferiprone can cause agranulocytosis that can lead to serious infections and death. Neutropenia may precede the development of agranulocytosis.
|
Agranulocytosis/Neutropenia
- Fatal agranulocytosis can occur with deferiprone use. Deferiprone can also cause neutropenia, which may foreshadow agranulocytosis. Measure the absolute neutrophil count (ANC) before starting deferiprone therapy and monitor the ANC weekly on therapy.
- Interrupt deferiprone therapy if neutropenia develops (ANC < 1.5 x 109/L).
- Interrupt deferiprone if infection develops, and monitor the ANC more frequently.
- Advise patients taking deferiprone to immediately interrupt therapy and report to their physician if they experience any symptoms indicative of infection.
- In pooled clinical trials, the incidence of agranulocytosis was 1.7% of patients. The mechanism of deferiprone-associated agranulocytosis is unknown. Agranulocytosis and neutropenia usually resolve upon discontinuation of deferiprone, but there have been reports of agranulocytosis leading to death.
- Implement a plan to monitor for and to manage agranulocytosis/neutropenia prior to initiating deferiprone treatment.
For neutropenia (ANC < 1.5 x 109/L and > 0.5 x 109/L)
- Instruct the patient to immediately discontinue deferiprone and all other medications with a potential to cause neutropenia.
- Obtain a complete blood cell (CBC) count, including a white blood cell (WBC) count corrected for the presence of nucleated red blood cells, an absolute neutrophil count (ANC), and a platelet count daily until recovery (ANC ≥ 1.5 x 109/L).
For agranulocytosis (ANC < 0.5 x 109/L)
- Consider hospitalization and other management as clinically appropriate.
- Do not resume deferiprone in patients who have developed agranulocytosis unless potential benefits outweigh potential risks. Do not rechallenge patients who develop neutropenia with deferiprone unless potential benefits outweigh potential risks.
Cardiac QT Syndrome
- A thorough QT study has not been conducted with deferiprone. One patient with a history of QT prolongation experienced Torsades de Pointes during therapy with deferiprone. Administer deferiprone with caution to patients who may be at increased risk of prolongation of the cardiac QT interval (e.g., those with congestive heart failure, bradycardia, use of a diuretic, cardiac hypertrophy, hypokalemia or hypomagnesemia). Instruct any patient taking deferiprone who experiences symptoms suggestive of an arrhythmia (such as palpitations, dizziness, lightheadedness, syncope, or seizures) to seek medical attention immediately.
Embryofetal toxicity
- Based on evidence of genotoxicity and developmental toxicity in animal studies, deferiprone can cause fetal harm when administered to a pregnant woman. In animal studies, administration of deferiprone during the period of organogenesis resulted in embryofetal death and malformations at doses lower than equivalent human clinical doses. If deferiprone is used during pregnancy or if the patient becomes pregnant while taking deferiprone, the patient should be apprised of the potential hazard to the fetus. Women of reproductive potential should be advised to avoid pregnancy when taking deferiprone.
Laboratory Tests
Serum liver enzyme activities
- In clinical studies, 7.5% of 642 subjects treated with deferiprone developed increased ALT values. Four (0.62%) deferiprone-treated subjects discontinued the drug due to increased serum ALT levels and 1 (0.16%) due to an increase in both ALT and AST.
- Monitor serum ALT values monthly during therapy with deferiprone, and consider interruption of therapy if there is a persistent increase in the serum transaminase levels.
Plasma Zinc concentration
- Decreased plasma zinc concentrations have been observed on deferiprone therapy. Monitor plasma zinc, and supplement in the event of a deficiency.
Adverse Reactions
Clinical Trials Experience
Because clinical trials are conducted under widely varying conditions, adverse reaction rates observed in the clinical trials of a drug cannot be directly compared to rates in the clinical trials of another drug and may not reflect the rates observed in practice.
- Adverse reaction information for deferiprone represents the pooled data collected from 642 patients who participated in single arm or active-controlled clinical studies.
- The most serious adverse reaction reported in clinical trials with deferiprone was agranulocytosis.
- The most common adverse reactions reported during clinical trials were chromaturia, nausea, vomiting, abdominal pain, alanine aminotransferase increased, arthralgia and neutropenia.
- The table below lists the adverse drug reactions that occurred in at least 1% of patients treated with deferiprone in clinical trials.

- Gastrointestinal symptoms such as nausea, vomiting, and abdominal pain were the most frequent adverse reactions reported by patients participating in clinical trials and led to the discontinuation of deferiprone therapy in 1.6% of patients.
- Chromaturia (reddish-brown discoloration of the urine) is a result of the excretion of the iron in the urine.
Postmarketing Experience
The following additional adverse reactions have been reported in patients receiving deferiprone. Because these reactions are reported voluntarily from a population of uncertain size, it is not always possible to reliably estimate their frequency or to establish a causal relationship to drug exposure.
- Blood and lymphatic system disorders: thrombocytosis, pancytopenia.
- Cardiac disorders: atrial fibrillation, cardiac failure.
- Congenital, familial and genetic disorders: hypospadias.
- Eye disorders: diplopia, papilledema, retinal toxicity.
- Gastrointestinal disorders: enterocolitis, rectal hemorrhage, gastric ulcer, pancreatitis, parotid gland enlargement.
- General disorders and administration site conditions: chills, pyrexia, edema peripheral, multi-organ failure.
- Hepatobiliary disorders: jaundice, hepatomegaly.
- Immune system disorders: anaphylactic shock, hypersensitivity.
- Infections and infestations: cryptococcal cutaneous infection, enteroviral encephalitis, pharyngitis, pneumonia, sepsis, furuncle, infection hepatitis, rash pustular, subcutaneous abscess.
- Investigations: blood bilirubin increased, blood creatinine phosphokinase increased.
- Metabolism and nutrition disorders: metabolic acidosis, dehydration.
- Musculoskeletal and connective tissue disorders: myositis, chondropathy, trismus.
- Nervous system disorders: cerebellar syndrome, cerebral hemorrhage, convulsion, gait disturbance, intracranial pressure increased, psychomotor skills impaired, pyramidal tract syndrome, somnolence.
- Psychiatric disorders: bruxism, depression, obsessive-compulsive disorder.
- Renal disorders: glycosuria, hemoglobinuria.
- Respiratory, thoracic and mediastinal disorders: acute respiratory distress syndrome, epistaxis, hemoptysis, pulmonary embolism.
- Skin, subcutaneous tissue disorders: hyperhidrosis, periorbital edema, photosensitivity reaction, pruritis, urticaria, rash, Henoch-Schönlein purpura.
- Vascular disorders: hypotension, hypertension.
Drug Interactions
Interactions with Foods, Vitamins and Antacids
Allow at least a 4-hour interval between deferiprone and other medications or supplements containing polyvalent cations such as iron, aluminum, and zinc
Drugs associated with neutropenia or agranulocytosis
Avoid concomitant use of deferiprone with other drugs known to be associated with neutropenia or agranulocytosis; however, if this is not possible, closely monitor the absolute neutrophil count.
UDP-glucuronosyltransferases (UGTs)
Deferiprone is primarily eliminated via metabolism to the 3-O-glucuronide. In vitro studies suggest that UDP glucuronosyltransferase (UGT) 1A6 is primarily responsible for the glucuronidation of deferiprone which can be reduced up to 78% in the presence of the UGT1A6 inhibitor phenylbutazone. However, the clinical significance of coadministration of deferiprone with a UGT1A6 inhibitor (e.g. diclofenac, probenecid, or silymarin (milk thistle)) on the systemic exposure of deferiprone has not been determined. Closely monitor patients for adverse reactions that may require downward dose titration or interruption when deferiprone is concomitantly administered with a UGT1A6 inhibitor.
Polyvalent cations
Concurrent use of deferiprone with foods, mineral supplements, and antacids that contain polyvalent cations has not been studied. However, since deferiprone has the potential to bind polyvalent cations (e.g., iron, aluminum, and zinc), allow at least a 4-hour interval between deferiprone and other medications (e.g., antacids), or supplements containing these polyvalent cations.
Use in Specific Populations
Pregnancy
Pregnancy Category (FDA): D Based on evidence of genotoxicity and developmental toxicity in animal studies, deferiprone can cause fetal harm when administered to a pregnant woman. In animal studies, administration of deferiprone during the period of organogenesis resulted in embryofetal death and malformations at doses lower than equivalent human clinical doses. There are no studies in pregnant women, and available human data are limited. If deferiprone is used during pregnancy or if the patient becomes pregnant while taking deferiprone, the patient should be apprised of the potential hazard to the fetus.
Skeletal and soft tissue malformations occurred in offspring of rats and rabbits that received deferiprone orally during organogenesis at the lowest doses tested (25 mg/kg per day in rats; 10 mg/kg per day in rabbits). These doses were equivalent to 3% to 4% of the maximum recommended human dose (MRHD) based on body surface area. No maternal toxicity was evident at these doses.
Embryofetal lethality and maternal toxicity occurred in pregnant rabbits given 100 mg/kg/day deferiprone orally during the period of organogenesis. This dose is equivalent to 32% of the MRHD based on body surface area.
Pregnancy Category (AUS):
There is no Australian Drug Evaluation Committee (ADEC) guidance on usage of Deferiprone in women who are pregnant.
Labor and Delivery
There is no FDA guidance on use of Deferiprone during labor and delivery.
Nursing Mothers
It is not known whether deferiprone is excreted in human milk. Because many drugs are excreted in human milk and because of the potential for adverse reactions in nursing infants from deferiprone, a decision should be made whether to discontinue nursing or to discontinue the drug, taking into account the importance of the drug to the mother.
Pediatric Use
The safety and effectiveness of deferiprone tablets for oral use in pediatric patients have not been established.
Geriatic Use
Safety and effectiveness in elderly individuals have not been established. In general, dose selection for an elderly patient should be cautious, usually starting at the low end of the dosing range, reflecting the greater frequency of decreased hepatic, renal, or cardiac function, and of concomitant disease or other drug therapy.
Gender
the influence of gender has not been established.
Race
the influence of race has not been established.
Renal Impairment
Deferiprone has not been evaluated in patients with renal impairment.
Hepatic Impairment
Deferiprone has not been conclusively evaluated in patients with hepatic impairment.
Females of Reproductive Potential and Males
A fertility and early embryonic development study of deferiprone was conducted in rats. Sperm counts, motility and morphology were unaffected by treatment with deferiprone. There were no effects observed on male or female fertility or reproductive function at the highest dose which was 25% of the MRHD based on body surface area.
Immunocompromised Patients
There is no FDA guidance one the use of Deferiprone in patients who are immunocompromised.
Administration and Monitoring
Administration
Oral
Monitoring
There is limited information regarding Deferiprone Monitoring in the drug label.
IV Compatibility
There is limited information regarding the compatibility of Deferiprone and IV administrations.
Overdosage
No cases of acute overdose have been reported. There is no specific antidote to deferiprone overdose.
Neurological disorders such as cerebellar symptoms, diplopia, lateral nystagmus, psychomotor slowdown, hand movements and axial hypotonia have been observed in children treated with 2.5 to 3 times the recommended dose for more than one year. The neurological disorders progressively regressed after deferiprone discontinuation.
Pharmacology
| |
Deferiprone
| |
| Systematic (IUPAC) name | |
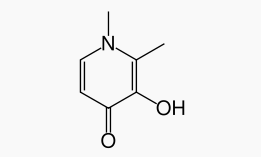
| 3-hydroxy-1,2-dimethylpyridin-4(1H)-one | |
| Identifiers | |
| CAS number | |
| ATC code | V03 |
| PubChem | |
| Chemical data | |
| Formula | Template:OrganicBox atomTemplate:OrganicBox atomTemplate:OrganicBoxTemplate:OrganicBoxTemplate:OrganicBoxTemplate:OrganicBoxTemplate:OrganicBoxTemplate:OrganicBoxTemplate:OrganicBoxTemplate:OrganicBoxTemplate:OrganicBoxTemplate:OrganicBoxTemplate:OrganicBoxTemplate:OrganicBox atomTemplate:OrganicBoxTemplate:OrganicBox atomTemplate:OrganicBoxTemplate:OrganicBoxTemplate:OrganicBoxTemplate:OrganicBoxTemplate:OrganicBoxTemplate:OrganicBoxTemplate:OrganicBox |
| Mol. mass | 139.152 g/mol |
| SMILES | & |
| Pharmacokinetic data | |
| Bioavailability | ? |
| Metabolism | Glucuronidation |
| Half life | 2 to 3 hours |
| Excretion | Renal (75 to 90% in 24 hours) |
| Therapeutic considerations | |
| Licence data |
, |
| Pregnancy cat. |
D(US) |
| Legal status |
[[Prescription drug|Template:Unicode-only]](US) |
| Routes | Oral |
Mechanism of Action
Deferiprone is a chelating agent with an affinity for ferric ion (iron III). Deferiprone binds with ferric ions to form neutral 3:1 (deferiprone:iron) complexes that are stable over a wide range of pH values. Deferiprone has a lower binding affinity for other metals such as copper, aluminum and zinc than for iron.
Structure
Deferiprone has the following structural formula:

Pharmacodynamics
No clinical studies were performed to assess the relationship between the dose of deferiprone and the amount of iron eliminated from the body.
QT/QTc Prolongation
No clinical studies of the effects of deferiprone on the cardiac QT interval have been performed in human subjects
Pharmacokinetics
Deferiprone is rapidly absorbed from the upper part of the gastrointestinal tract, appearing in the blood within 5 to 10 minutes of oral administration. Peak serum concentrations occur approximately 1 hour after a single dose in fasted healthy subjects and patients, and up to 2 hours after a single dose in the fed state. Administration with food decreased the Cmax of deferiprone by 38% and the AUC by 10%. While a food effect cannot be ruled out, the magnitude of the exposure change does not warrant dose adjustment.
In healthy subjects, the mean maximum concentration (Cmax) of deferiprone in serum was 20 mcg/mL, and the mean total area under the concentration-time curve (AUC) was 53 mcg∙h/mL following oral administration of a 1,500 mg dose of deferiprone tablets in the fasting state. Dose proportionality over the labeled dosage range of 25 to 33 mg/kg three times per day (75 to 99 mg/kg per day) has not been studied. The elimination half life (t1/2) of deferiprone was 1.9 hours. The accumulation of deferiprone and its glucuronide metabolite at the highest approved dosage level of 33 mg/kg three times per day has not been studied. The volume of distribution of deferiprone is 1.6 L/kg in thalassemia patients, and approximately 1 L/kg in healthy subjects. The plasma protein binding of deferiprone in humans is less than 10%.
In humans, the majority of the deferiprone is metabolized, primarily by UGT1A6. The contribution of extrahepatic (e.g., renal) UGT1A6 is unknown. The major metabolite of deferiprone is the 3-O-glucuronide, which lacks iron binding capability. Peak serum concentration of the glucuronide occurs 2 to 4 hours after administration of deferiprone in fasting subjects.
More than 90% of deferiprone is eliminated from plasma within 5 to 6 hours of ingestion. Following oral administration, 75% to 90% is recovered in the urine in the first 24 hours, primarily as metabolite.
Nonclinical Toxicology
Carcinogenesis and Mutagenesis
Carcinogenicity studies have not been conducted with deferiprone. However, in view of the genotoxicity results, and the findings of mammary gland hyperplasia and mammary gland tumors in rats treated with deferiprone in the 52-week toxicology study, tumor formation in carcinogenicity studies must be regarded as likely.
Deferiprone was positive in a mouse lymphoma cell assay in vitro. Deferiprone was clastogenic in an in vitro chromosomal aberration test in mice and in a chromosomal aberration test in Chinese Hamster Ovary cells. Deferiprone given orally or intraperitoneally was clastogenic in a bone marrow micronucleus assay in non-iron-loaded mice. A micronucleus test was also positive when mice predosed with iron dextran were treated with deferiprone. Deferiprone was not mutagenic in the Ames bacterial reverse mutation test.
Clinical Studies
In a prospective, planned, pooled analysis of patients from several studies, the efficacy of deferiprone was assessed in transfusion-dependent iron overload patients in whom previous iron chelation therapy had failed or was considered inadequate due to poor tolerance. The main criterion for chelation failure was serum ferritin >2,500 mcg/L before treatment with deferiprone. deferiprone therapy (35-99 mg/kg/day) was considered successful in individual patients who experienced a ≥20% decline in serum ferritin within one year of starting therapy.
Data from a total of 236 patients were analyzed. Of the 224 patients with thalassemia who received deferiprone monotherapy and were eligible for serum ferritin analysis, 105 (47%) were male and 119 (53%) were female. The mean age of these patients was 18.2 years.
For the patients in the analysis, the endpoint of at least a 20% reduction in serum ferritin was met in 50% (of 236 subjects), with a 95% confidence interval of 43% to 57%.
A small number of patients with thalassemia and iron overload were assessed by measuring the change in the number of milliseconds (ms) in the cardiac MRI T2* value before and after treatment with deferiprone for one year. There was an increase in cardiac MRI T2* from a mean at baseline of 11.8 ± 4.9 ms to a mean of 15.1 ± 7.0 ms after approximately one year of treatment. The clinical significance of this observation is not known.
How Supplied
- Deferiprone 500 mg tablets
- 100 tablets (NDC 52609-0006-1)
Storage
Store at 20º to 25ºC (68º to 77ºF)
Images
Drug Images
{{#ask: Page Name::Deferiprone |?Pill Name |?Drug Name |?Pill Ingred |?Pill Imprint |?Pill Dosage |?Pill Color |?Pill Shape |?Pill Size (mm) |?Pill Scoring |?NDC |?Drug Author |format=template |template=DrugPageImages |mainlabel=- |sort=Pill Name }}
Package and Label Display Panel

{{#ask: Label Page::Deferiprone |?Label Name |format=template |template=DrugLabelImages |mainlabel=- |sort=Label Page }}
Patient Counseling Information
- Inform patients of the risks of developing agranulocytosis and instruct them to immediately interrupt therapy and report to their physician if they experience any symptoms of infection such as fever, sore throat or flu-like symptoms.
- Advise patients that the amount of deferiprone prescribed is based on body weight and on the therapeutic goal (reduction or stabilization of the body iron load).
- Advise patients to take the first dose of deferiprone in the morning, the second dose at midday, and the third dose in the evening. Clinical experience suggests that taking deferiprone with meals may reduce nausea. If a dose of this medicine has been missed, take as soon as possible. However, if it is almost time for the next dose, skip the missed dose and go back to the regular dosing schedule. Do not catch-up or double doses.
- Advise patients to contact their physician in the event of overdose.
- Inform patients that their urine might show a reddish/brown discoloration due to the excretion of the iron-deferiprone complex. This is a very common sign of the desired effect of deferiprone, and it is not harmful.
- Counsel women of reproductive potential to avoid pregnancy while taking deferiprone. Advise patients to immediately notify their physician if they become pregnant, or if they plan to become pregnant during therapy.
- Inform patients that they should not breast feed while taking deferiprone.
- Inform patients that if they experience palpitations, dizziness, lightheadedness, syncope, or seizures to immediately seek medical attention.
Precautions with Alcohol
Alcohol-Deferiprone interaction has not been established. Talk to your doctor about the effects of taking alcohol with this medication.
Brand Names
- Ferriprox [1]
Look-Alike Drug Names
There is limited information regarding Deferiprone Look-Alike Drug Names in the drug label.
Drug Shortage Status
Price
References
The contents of this FDA label are provided by the National Library of Medicine.
{{#subobject:
|Label Page=Deferiprone |Label Name=Deferiprone 500 mg.png
}}