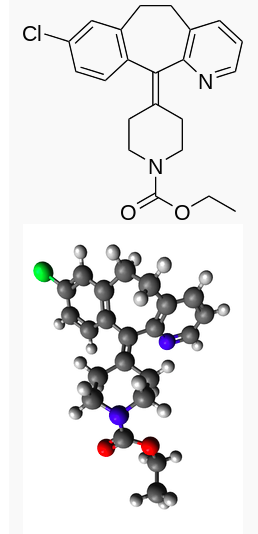
Loratadine
Editor-In-Chief: C. Michael Gibson, M.S., M.D. [1]; Associate Editor(s)-in-Chief: Alberto Plate [2]
Disclaimer
WikiDoc MAKES NO GUARANTEE OF VALIDITY. WikiDoc is not a professional health care provider, nor is it a suitable replacement for a licensed healthcare provider. WikiDoc is intended to be an educational tool, not a tool for any form of healthcare delivery. The educational content on WikiDoc drug pages is based upon the FDA package insert, National Library of Medicine content and practice guidelines / consensus statements. WikiDoc does not promote the administration of any medication or device that is not consistent with its labeling. Please read our full disclaimer here.
Overview
Loratadine is an antihistamine that is FDA approved for the treatment of runny nose, sneezing, itchy, watery eyes, itching of the nose or throat. Common adverse reactions include xerostomia, headache, somnolence and fatigue.
Adult Indications and Dosage
FDA-Labeled Indications and Dosage (Adult)
Seasonal allergic rhinitis
- Dose: 10 mg PO daily
Idiopathic urticaria
- Dose: 10 mg PO daily
Off-Label Use and Dosage (Adult)
Guideline-Supported Use
There is limited information regarding Off-Label Guideline-Supported Use of Loratadine in adult patients.
Non–Guideline-Supported Use
Asthma
Eosinophilic nonallergic rhinitis
Pediatric Indications and Dosage
FDA-Labeled Indications and Dosage (Pediatric)
Seasonal allergic rhinitis
- Dose (2-5 years): 5 mg PO daily
- Dose (>6 years): 10 mg PO daily
Idiopathic urticaria
- Dose 2-5 years: 5 mg PO daily
- Dose >6 years: 10 mg PO daily
Off-Label Use and Dosage (Pediatric)
Guideline-Supported Use
There is limited information regarding Off-Label Guideline-Supported Use of Loratadine in pediatric patients.
Non–Guideline-Supported Use
Asthma
- <30 kg: 10 mg PO daily
- >30 kg: 20 mg PO daily
Contraindications
- Hypersensitivity to loratadine
Warnings
- Do not use if you have ever had an allergic reaction to this product or any of its ingredients
- Ask a doctor before use if you have liver or kidney disease. Your doctor should determine if you need a different dose.
- When using this product do not take more than directed. Taking more than directed may cause drowsiness.
- Stop use and ask a doctor is an allergic reaction to this product occurs. Seek medical help right away.
- If pregnant or breast-feeding, ask a health professional before use.
- Keep out of reach of children.
- In case of overdose, get medical help or contact a Poison Control Center right away.
Adverse Reactions
Clinical Trials Experience
Dermatologic
Gastrointestinal
Hepatic
Neurologic
Respiratory
- Epistaxis
- Pharyngitis
- Upper respiratory infections
- Viral disease
- Wheezing
Other
Postmarketing Experience
There is limited information regarding Loratadine Postmarketing Experience in the drug label.
Drug Interactions
- Amiodarone: QT interval prolongation and Torsade de Pointes have been reported with the co-administration of loratadine and amiodarone.
- Carbamazepine: Loratadine increase plasma concentration of carbamazepine.
- Cimetidine: increases plasma concentrations of loratadine
- Ketoconazole: increases plasma concentrations of loratadine
- Mepenzolate
- Morphine: increases risk of paralytic ileus
- Morphine sulfate liposome: increases risk of paralytic ileus
- Oximorphone
- Umeclidinium
Use in Specific Populations
Pregnancy
- Ask a health professional before use.
Pregnancy Category (AUS): B1
There is no Australian Drug Evaluation Committee (ADEC) guidance on usage of Loratadine in women who are pregnant.
Labor and Delivery
There is no FDA guidance on use of Loratadine during labor and delivery.
Nursing Mothers
- Ask a health professional before use.
Pediatric Use
- In case of overdose, get medical help or contact a Poison Control Center right away. (1-800-222-1222)
Geriatic Use
There is no FDA guidance on the use of Loratadine in geriatric settings.
Gender
There is no FDA guidance on the use of Loratadine with respect to specific gender populations.
Race
There is no FDA guidance on the use of Loratadine with respect to specific racial populations.
Renal Impairment
There is no FDA guidance on the use of Loratadine in patients with renal impairment.
Hepatic Impairment
There is no FDA guidance on the use of Loratadine in patients with hepatic impairment.
Females of Reproductive Potential and Males
There is no FDA guidance on the use of Loratadine in women of reproductive potentials and males.
Immunocompromised Patients
There is no FDA guidance one the use of Loratadine in patients who are immunocompromised.
Administration and Monitoring
Administration
Oral
Monitoring
There is limited information regarding Loratadine Monitoring in the drug label.
IV Compatibility
There is limited information regarding the compatibility of Loratadine and IV administrations.
Overdosage
There is limited information regarding Loratadine overdosage. If you suspect drug poisoning or overdose, please contact the National Poison Help hotline (1-800-222-1222) immediately.
Pharmacology
Mechanism of Action
Loratadine is a tricyclic antihistamine, which acts as a selective inverse agonist of peripheral histamine H1-receptors.
Structure
There is limited information regarding Loratadine Structure in the drug label.
Pharmacodynamics
There is limited information regarding Loratadine Pharmacodynamics in the drug label.
Pharmacokinetics
Loratadine is given orally, is well absorbed from the gastrointestinal tract, and has rapid first-pass hepatic metabolism; it is metabolized by isoenzymes of the cytochrome P450 system, including CYP3A4, CYP2D6, and, to a lesser extent, several others.[3][4] Loratadine is almost totally (97–99%) bound to plasma proteins. Its metabolite desloratadine, which is largely responsible for the antihistaminergic effects, binds to plasma proteins by 73–76%.
Nonclinical Toxicology
There is limited information regarding Loratadine Nonclinical Toxicology in the drug label.
Clinical Studies
There is limited information regarding Loratadine Clinical Studies in the drug label.
How Supplied
- Supplied as tablets of 10 mg in 30 tablet carton and 40 tablet bottle carton.
Storage
- Store at 20°-25°C (68°-77°F)
Images
Drug Images
{{#ask: Page Name::Loratadine |?Pill Name |?Drug Name |?Pill Ingred |?Pill Imprint |?Pill Dosage |?Pill Color |?Pill Shape |?Pill Size (mm) |?Pill Scoring |?NDC |?Drug Author |format=template |template=DrugPageImages |mainlabel=- |sort=Pill Name }}
Package and Label Display Panel
{{#ask: Label Page::Loratadine |?Label Name |format=template |template=DrugLabelImages |mainlabel=- |sort=Label Page }}
Patient Counseling Information
There is limited information regarding Loratadine Patient Counseling Information in the drug label.
Precautions with Alcohol
Alcohol-Loratadine interaction has not been established. Talk to your doctor about the effects of taking alcohol with this medication.
Brand Names
- Alavert
- Claritin
- Claritin Reditabs
- Clearatadine
- Triaminic Allerchews
- Children's Clear-Atadine
- Children's Dimetapp ND Allergy
- Children's Claritin
Look-Alike Drug Names
There is limited information regarding Loratadine Look-Alike Drug Names in the drug label.
Drug Shortage Status
Price
References
The contents of this FDA label are provided by the National Library of Medicine.
- ↑ Menardo JL, Horak F, Danzig MR, Czarlewski W (1997). "A review of loratadine in the treatment of patients with allergic bronchial asthma". Clin Ther. 19 (6): 1278–93, discussion 1523-4. PMID 9444440.
- ↑ Corren J, Harris AG, Aaronson D, Beaucher W, Berkowitz R, Bronsky E; et al. (1997). "Efficacy and safety of loratadine plus pseudoephedrine in patients with seasonal allergic rhinitis and mild asthma". J Allergy Clin Immunol. 100 (6 Pt 1): 781–8. PMID 9438487.
- ↑ Nelson, Wendel L. (2002). "Antihistamines and related antiallergic and antiulcer agents". In Williams, David H.; Foye, William O.; Lemke, Thomas L. Foye's principles of medicinal chemistry. Hagerstown, MD: Lippincott Williams & Wilkins. p. 805. ISBN 0-683-30737-1. Retrieved on 1 December 2009 through Google Book Search.
- ↑ Ghosal A, Gupta S, Ramanathan R; et al. (August 2009). "Metabolism of loratadine and further characterization of its in vitro metabolites". Drug Metab Lett. 3 (3): 162–70. doi:10.2174/187231209789352067. PMID 19702548.
{{#subobject:
|Page Name=Loratadine
|Pill Name=
|Drug Name=
|Pill Ingred=|+sep=;
|Pill Imprint=
|Pill Dosage={{{dosageValue}}} {{{dosageUnit}}}
|Pill Color=|+sep=;
|Pill Shape=
|Pill Size (mm)=
|Pill Scoring=
|Pill Image=
|Drug Author=
|NDC=
}}
{{#subobject:
|Label Page=Loratadine |Label Name=Loratadine FDA panel2.png
}}
{{#subobject:
|Label Page=Loratadine |Label Name=Loratadine FDA panel1 .png
}}
{{#subobject:
|Label Page=Loratadine |Label Name=Loratadine Package2.png
}}
{{#subobject:
|Label Page=Loratadine |Label Name=Loratadine Package1.png
}}