Calcitriol (topical)
Editor-In-Chief: C. Michael Gibson, M.S., M.D. [1]; Associate Editor(s)-in-Chief: Kiran Singh, M.D. [2]
Disclaimer
WikiDoc MAKES NO GUARANTEE OF VALIDITY. WikiDoc is not a professional health care provider, nor is it a suitable replacement for a licensed healthcare provider. WikiDoc is intended to be an educational tool, not a tool for any form of healthcare delivery. The educational content on WikiDoc drug pages is based upon the FDA package insert, National Library of Medicine content and practice guidelines / consensus statements. WikiDoc does not promote the administration of any medication or device that is not consistent with its labeling. Please read our full disclaimer here.
Overview
Calcitriol (topical) is a fat-soluble vitamin that is FDA approved for the treatment of mild to moderate plaque psoriasis. Common adverse reactions include lab test abnormality, urine abnormality, hypercalciuria, and pruritus.
Adult Indications and Dosage
FDA-Labeled Indications and Dosage (Adult)
Indication
- Calcitriol Ointment is indicated for the topical treatment of mild to moderate plaque psoriasis in adults 18 years and older.
Limitations of Use
- Calcitriol Ointment should not be applied to the eyes, lips, or facial skin.
Dosage
- Apply Calcitriol Ointment to affected areas twice daily, morning and evening. The maximum weekly dose should not exceed 200 grams. Calcitriol Ointment is not for oral, ophthalmic or intravaginal use.
DOSAGE FORMS AND STRENGTHS
- Each gram of ointment contains 3 micrograms (mcg/g) of calcitriol.
Off-Label Use and Dosage (Adult)
Guideline-Supported Use
There is limited information regarding Off-Label Guideline-Supported Use of Calcitriol (topical) in adult patients.
Non–Guideline-Supported Use
There is limited information regarding Off-Label Non–Guideline-Supported Use of Calcitriol (topical) in adult patients.
Pediatric Indications and Dosage
FDA-Labeled Indications and Dosage (Pediatric)
There is limited information regarding FDA-Labeled Use of Calcitriol (topical) in pediatric patients.
Off-Label Use and Dosage (Pediatric)
Guideline-Supported Use
There is limited information regarding Off-Label Guideline-Supported Use of Calcitriol (topical) in pediatric patients.
Non–Guideline-Supported Use
There is limited information regarding Off-Label Non–Guideline-Supported Use of Calcitriol (topical) in pediatric patients.
Contraindications
- None
Warnings
Effects on Calcium Metabolism
- In controlled clinical trials with Calcitriol Ointment, among subjects having laboratory monitoring, hypercalcemia was observed in 24% (18/74) of subjects exposed to active drug and in 16% (13/79) of subjects exposed to vehicle. However, the increases in calcium and albumin-adjusted calcium levels were less than 10% above the upper limit of normal.
- If aberrations in parameters of calcium metabolism occur, treatment should be discontinued until these parameters have normalized. The effects of Calcitriol Ointment on calcium metabolism following treatment durations greater than 52 weeks have not been evaluated. Increase absorption may occur with occlusive use.
Ultraviolet Light Exposure
- Animal data suggest that the vehicle of calcitriol Ointment may enhance the ability of ultraviolent radiation (UVR) to induce skin tumors [see Carcinogenesis, Mutagenesis, Impairment of Fertility (13.1)] Subjects who apply Calcitriol Ointment to exposed skin should avoid excessive exposure to the treated areas to either natural or artificial sunlight, including tanning booths and sun lamps. Physicians may wish to limit or avoid use of phototherapy in patients who use Calcitriol Ointment.
Unevaluated Uses
- The safety and effectiveness of Calcitriol Ointment in patients with known or suspected disorders of calcium metabolism have not been evaluated. The safety and effectiveness of Calcitriol Ointment in patients with erythrodermic, exfoliative, or pustular psoriasis have not been evaluated.
Adverse Reactions
Clinical Trials Experience
- Because clinical studies are conducted under widely varying conditions, adverse reaction rates observed in the clinical studies of a drug cannot be directly compared to rates in the clinical studies of another drug and may not reflect the rate observed in practice.
Clinical Studies Experience
- Calcitriol Ointment was studied in two vehicle-controlled studies (419 subjects), and in one open label study (324 subjects). The table below describes exposure to Calcitriol Ointment in 743 subjects, including 239 exposed for 6 months and 116 exposed for one year.
- Four hundred and nineteen subjects were treated with Calcitriol Ointment twice daily for 8 weeks. The population included subjects ages 13 to 87, males (284) and females (135), Caucasians (372) and non-Caucasians (47); with mild (105) to moderate (313) chronic plaque psoriasis.

- Among subjects having laboratory monitoring, hypercalcemia was observed in 24% (18/74) of subjects exposed to active drug and in 16% (13/79) of subjects exposed to vehicle, however the elevation were less than 10% above the upper limit of normal.
- The open label study enrolled 324 subjects with psoriasis who were then treated for up to 52 weeks. Adverse events reported at a rate of greater than or equal to 3% of subjects treated with Calcitriol Ointment were lab test abnormality (8%), urine abnormality (4%), psoriasis (4%), hyperciuria (3%), and pruritus (3%). Kidney stones were reported in 3 subjects and confirmed in two.
Postmarketing Experience
- The following adverse reactions have been identified during the world-wide post-approval use of Calcitriol Ointment: acute blistering dermatitis, erythema, pruritus, skin burning sensation, and skin discomfort. Because these reactions were reported voluntarily from a population of uncertain size, it is not always possible to reliably estimate their frequency or establish a causal relationship to drug exposure.
Drug Interactions
- Calcitriol Ointment should be used with caution in patients receiving medications known to increase the serum calcium level, such as thiazide diuretics. Caution should also be exercised in patients receiving calcium supplements or high doses of vitamin D.
Use in Specific Populations
Pregnancy
Pregnancy Category (FDA): Pregnancy
Teratogenic Effects: Pregnancy Category C.
- Calcitriol Ointment contains calcitriol which has been shown to be fetotoxic. There are no adequate and well-controlled studies for Calcitriol Ointment in pregnant women. Calcitriol Ointment should be used during pregnancy only if the potential benefit to the patient justifies the potential risk to the fetus.
- Teratogenicity studies with calcitriol were performed in which rats were treated orally at dosages up to 0.9 mcg/kg/day (5.4 mcg/m2/day) and in which rabbits received topical application of calcitriol ointment (3 ppm) to 6.4% of the body surface area. No effects on reproductive or fetal parameters were observed in rats. In rabbits, topically applied calcitriol induced a significantly elevated mean post-implantation loss and an increased incidence of minor skeletal abnormalities due to retarded ossification of the pubic bones. A slightly increased incidence of skeletal variation (extra 13th rib, reduced ossification of epiphyses) was also observed. These effects may have been secondary to maternal toxicity. Based on the recommended human dose and instructions for use, it is not possible to calculate human dose equivalents for animal exposures in these studies.
Pregnancy Category (AUS):
There is no Australian Drug Evaluation Committee (ADEC) guidance on usage of Calcitriol (topical) in women who are pregnant.
Labor and Delivery
There is no FDA guidance on use of Calcitriol (topical) during labor and delivery.
Nursing Mothers
- It is not known whether calcitriol is excreted in human milk. Because many drugs are excreted in human milk, caution should be exercised when calcitriol Ointment is administered to a nursing woman.
Pediatric Use
- Safety and effectiveness in pediatric patients have not been established.
Geriatic Use
- Clinical studies of Calcitriol Ointment did not include sufficient numbers of subjects aged 65 and over to determine whether they respond differently from younger subjects. Other reported experience has not identified differences in responses between the elderly and younger patients.
Gender
There is no FDA guidance on the use of Calcitriol (topical) with respect to specific gender populations.
Race
There is no FDA guidance on the use of Calcitriol (topical) with respect to specific racial populations.
Renal Impairment
There is no FDA guidance on the use of Calcitriol (topical) in patients with renal impairment.
Hepatic Impairment
There is no FDA guidance on the use of Calcitriol (topical) in patients with hepatic impairment.
Females of Reproductive Potential and Males
There is no FDA guidance on the use of Calcitriol (topical) in women of reproductive potentials and males.
Immunocompromised Patients
There is no FDA guidance one the use of Calcitriol (topical) in patients who are immunocompromised.
Administration and Monitoring
Administration
- Topical
Monitoring
There is limited information regarding Monitoring of Calcitriol (topical) in the drug label.
IV Compatibility
There is limited information regarding IV Compatibility of Calcitriol (topical) in the drug label.
Overdosage
- Topically applied calcitriol can be absorbed in sufficient amounts to produce systemic effects
Pharmacology
Mechanism of Action
- The mechanism of action of calcitriol in the treatment of psoriasis has not been established.
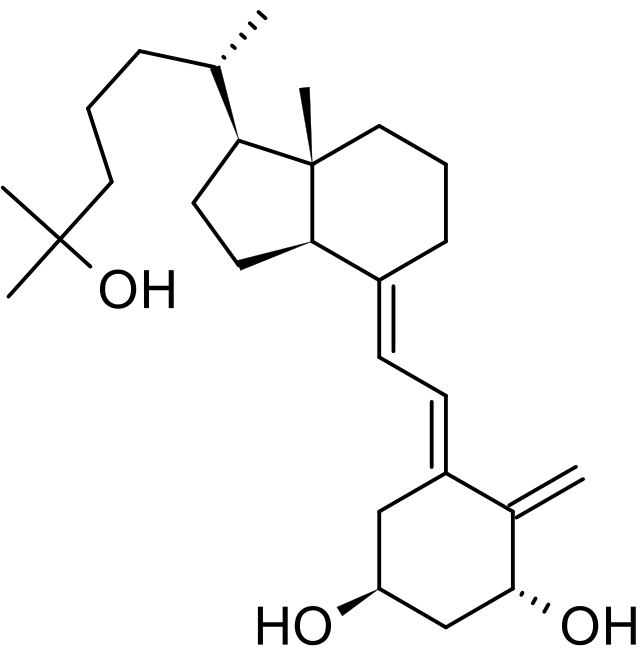
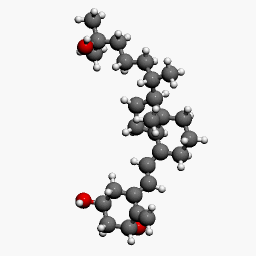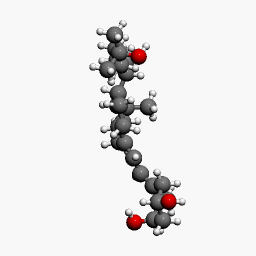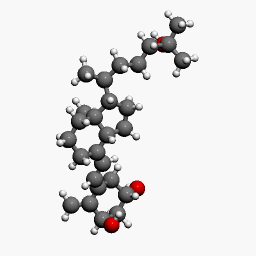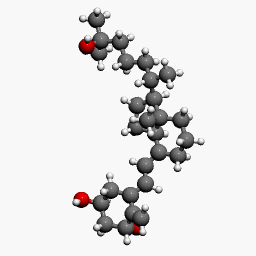
Structure
Calcitriol Ointment, 3 mcg/g is a vitamin D analog intended for topical application to the skin. The chemical name of the active ingredient is (5Z,7E)-9, 10-secocholesta-5,7,10(19)-triene-1α, 3β,25-triol. The structural formula is:

- Calcitriol is a white or almost white crystalline solid. It is practically insoluble in water, soluble in alcohol and in fatty oils. The molecular formula is C27H44O3, and the molecular weight is 416.64.
- Calcitriol Ointment is a translucent ointment containing 3 mcg/g (0.0003% w/w) of calcitriol, packaged in aluminum tubes with screw caps. Other components of the ointment are mineral oil, dl-α-tocopherol, and white petrolatum.
Pharmacodynamics
There is limited information regarding Pharmacodynamics of Calcitriol (topical) in the drug label.
Pharmacokinetics
- The systemic exposure of calcitriol was assessed in subjects with chronic, plaque psoriasis. In the pivotal pharmacokinetic/pharmacodynamic study, calcitriol ointment 3 mcg/g, was applied twice daily for 21 days (for a total dose of 30 g/day) to 35% of the body surface area (psoriatic + surrounding healthy skin) of subjects with at least 25% of body surface area involvement. At Day 21, the geometric mean plasma concentration values of Cmax increased by approximately 36% over baseline and the geometric mean value of AUC(0-12hr) increased by 44%. There was no correlation between the elevated calcitriol levels and the pharmacodynamic parameters or serum albumin adjusted calcium, serum phosphorus, urinary calcium and urinary phosphorus.
Nonclinical Toxicology
Carcinogenesis, Mutagenesis, Impairment of Fertility
- When calcitriol was applied topically to mice for up to 24 months, no significant changes in tumor incidence were observed. Concentrations of calcitriol in ointment base of 0 (vehicle control), 0.3, 0.6 and 1.0 ppm were evaluated.
- A two-year carcinogenicity study was conducted in which calcitriol was orally administered to rats at dosages of approximately0.005, 0.03, and 0.1 mcg/kg/day (0.03, 0.18, and 0.6 mcg/m2/day, respectively). The incidence of benign pheochromocytomas was significantly increased in female rats. No other significant differences in tumor incidence data were observed.
- In a study in which albino hairless mice were exposed to both ultra-violet radiation (UVR) and topically applied calcitriol ointment, a reduction in the time required for UVR to induce the formation of skin tumors was observed in all groups that received the ointment base, including the vehicle-treated control group, relative to animals that received no ointment but which were exposed to UVR. The time required for UVR to induce the formation of skin tumors did not differ between animals that received plain vehicle and those that recieved vehicle that contained calcitriol. Concentrations of calcitriol in ointment base of 0 (vehicle control), 0.3, 0.6, and 1.0 ppm were evaluated. These data suggest that the vehicle of Calcitriol Ointment may enhance the ability of UVR to induce skin tumors.
- Calcitriol did not elicit genotoxic effects in the mouse lymphoma TK locus assay.
- Studies in which male and female rats received oral doses of calcitriol of up to 0.6 mcg/kg/day (3.6 mcg/m2/day) indicated no impairment of fertility or general reproductive performances.
- Based upon the recommended human dose and instructions for use, it is not possible to calculate human dose equivalents for animal exposure in these studies.
Clinical Studies
- In two, multicenter, double-blind, vehicle-controlled studies, a total of 839 subjects with psoriasis rated “mild” or “moderate” using an investigator global assessment scale were tested twice daily for 8 weeks. Subjects were randomized in a 1:1 ratio to receive either Calcitriol Ointment or vehicle ointment. The mean age of subjects was 48 years and 66% were male; most subjects were rated “moderate” at baseline.
- Success was defined as "Clear or Minimal" (up to light red or pink coloration, surface dryness with some white coloration, and slight elevation above normal skin) with at least 2-grade change from baseline. The success rates are displayed in the table.

How Supplied
- Calcitriol Ointment 3 mcg/g is available in collapsible aluminum tubes of the following package sizes:
- 100 g tube (NDC 45802-608-01)
Storage
- Store at controlled room temperature 68° - 77°F (20° - 25°C) with excursions permitted between 59° - 86°F (15° - 30°C).Do not freeze or refrigerate.
Images
Drug Images
{{#ask: Page Name::Calcitriol (topical) |?Pill Name |?Drug Name |?Pill Ingred |?Pill Imprint |?Pill Dosage |?Pill Color |?Pill Shape |?Pill Size (mm) |?Pill Scoring |?NDC |?Drug Author |format=template |template=DrugPageImages |mainlabel=- |sort=Pill Name }}
Package and Label Display Panel


{{#ask: Label Page::Calcitriol (topical) |?Label Name |format=template |template=DrugLabelImages |mainlabel=- |sort=Label Page }}
Patient Counseling Information
- This information is intended to aid in the safe and effective use of this medication. It is not a disclosure of all possible adverse or intended effects. Patients using Calcitriol Ointment should receive the following information:
Instructions for Use
- This medication is to be used as directed by the physician. It is for external use only. This medication is to be applied only to areas of the skin affected by psoriasis, as directed. It should be gently rubbed into the skin so that no medication remains visible.
Adverse Reactions
- Patients should report any signs of adverse reactions to their physician.
Precautions with Alcohol
- Alcohol-Calcitriol (topical) interaction has not been established. Talk to your doctor about the effects of taking alcohol with this medication.
Brand Names
- CALCITRIOL®[1]
Look-Alike Drug Names
There is limited information regarding Calcitriol (topical) Look-Alike Drug Names in the drug label.
Drug Shortage Status
Price
References
The contents of this FDA label are provided by the National Library of Medicine.
{{#subobject:
|Page Name=Calcitriol (topical)
|Pill Name=No image.jpg
|Drug Name=
|Pill Ingred=|+sep=;
|Pill Imprint=
|Pill Dosage={{{dosageValue}}} {{{dosageUnit}}}
|Pill Color=|+sep=;
|Pill Shape=
|Pill Size (mm)=
|Pill Scoring=
|Pill Image=
|Drug Author=
|NDC=
}}
{{#subobject:
|Label Page=Calcitriol (topical) |Label Name=Calcitriol (topical)11.png
}}
{{#subobject:
|Label Page=Calcitriol (topical) |Label Name=Calcitriol (topical)11.png
}}