CLONIDINE patch patient counseling information
Editor-In-Chief: C. Michael Gibson, M.S., M.D. [1]; Associate Editor(s)-in-Chief: Pratik Bahekar, MBBS [2]
For patient information, click here.
Patient Counseling Information
Patients should be cautioned against interruption of Clonidine Transdermal System therapy without their physician’s advice.
Since patients may experience a possible sedative effect, dizziness, or accommodation disorder with use of clonidine, caution patients about engaging in activities such as driving a vehicle or operating appliances or machinery. Also, inform patients that this sedative effect may be increased by concomitant use of alcohol, barbiturates, or other sedating drugs.
Patients who wear contact lenses should be cautioned that treatment with Clonidine Transdermal System may cause dryness of eyes.
Patients should be instructed to consult their physicians promptly about the possible need to remove the patch if they observe moderate to severe localized erythema and/or vesicle formation at the site of application or generalized skin rash.
If a patient experiences isolated, mild localized skin irritation before completing 7 days of use, the system may be removed and replaced with a new system applied to a fresh skin site.
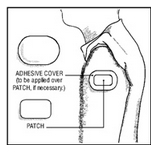
If the system should begin to loosen from the skin after application, the patient should be instructed to place the adhesive cover directly over the system to ensure adhesion during its 7-day use.
Used Clonidine Transdermal System patches contain a substantial amount of their initial drug content which may be harmful to infants and children if accidentally applied or ingested. THEREFORE, PATIENTS SHOULD BE CAUTIONED TO KEEP BOTH USED AND UNUSED CLONIDINE TRANSDERMAL SYSTEM PATCHES OUT OF THE REACH OF CHILDREN. After use, Clonidine Transdermal System should be folded in half with the adhesive sides together and discarded away from children’s reach.
Instructions for use, storage and disposal of the system are provided at the end of this monograph. These instructions are also included in each box of Clonidine Transdermal System.
Clonidine Transdermal System, USP
(Read the following instructions carefully before using this medication. If you have any questions, please consult with your doctor.)
General Information
Clonidine Transdermal System is a peach colored, rectangular PATCH with rounded corners, containing an active blood-pressure-lowering medication. It is designed to deliver the drug into the body through the skin smoothly and consistently for one full week. Normal exposure to water, as in showering, bathing, and swimming, should not affect the PATCH.
The optional ivory ADHESIVE COVER should be applied directly over the PATCH, should the PATCH begin to separate from the skin. The ADHESIVE COVER ensures that the PATCH sticks to the skin. The Clonidine Transdermal System PATCH must be replaced with a new one on a fresh skin site if the one in use significantly loosens or falls off.
 |
How to Apply the Clonidine Transdermal System PATCH
1) Apply the peach colored, rectangular PATCH with rounded corners, once a week, preferably at a convenient time on the same day of the week (i.e., prior to bedtime on Tuesday of week one; prior to bedtime on Tuesday of week two, etc.).
Each box of Clonidine Transdermal System contains two types of pouches:
 |
2) Select a hairless area such as on the upper, outer arm or upper chest. The area chosen should be free of cuts, abrasions, irritation, scars or calluses and should not be shaved before applying the Clonidine Transdermal System PATCH. Do not place the Clonidine Transdermal System PATCH on skin folds or under tight undergarments, since premature loosening may occur.
3) Wash hands with soap and water and thoroughly dry them.
4) Clean the area chosen with soap and water. Rinse and wipe dry with a clean, dry tissue.
5) Select the pouch labeled Clonidine Transdermal System, USP and open it as illustrated in Figure 3. Remove the contents of the pouch and discard the additional pieces of clear protective film above and below the PATCH.
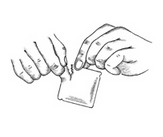 |
6) Remove the clear plastic protective backing from the peach colored, rectangular PATCH by gently peeling off one half of the backing at a time as shown in Figure 4. Avoid touching the sticky side of the Clonidine Transdermal System PATCH.
 |
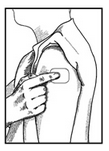
7) Place the Clonidine Transdermal System PATCH on the prepared skin site (sticky side down) by applying firm pressure over the PATCH to ensure good contact with the skin, especially around the edges (Figure 5). Discard the clear plastic protective backing and wash your hands with soap and water to remove any drug from your hands.
 |
8) After one week, remove the old PATCH and discard it (refer to Instructions for Disposal). After choosing a different skin site, repeat instructions 2 through 7 for the application of your next Clonidine Transdermal System PATCH.
What to do if your Clonidine Transdermal System PATCH becomes loose while wearing:
How to Apply the ADHESIVE COVER
NOTE: The ivory ADHESIVE COVER does not contain any drug and should not be used alone. The COVER should be applied directly over the Clonidine Transdermal System PATCH only if the PATCH begins to separate from the skin, thereby ensuring that it sticks to the skin for 7 full days.
1) Wash hands with soap and water and thoroughly dry them.
2) Using a clean, dry tissue, make sure that the area around the rectangular, peach Clonidine Transdermal System PATCH is clean and dry. Press gently on the Clonidine Transdermal System PATCH to ensure that the edges are in good contact with the skin.
3) Take the ivory ADHESIVE COVER (Figure 6) from the plain white pouch and remove the paper liner backing from the COVER.
 |
4) Carefully center the ivory ADHESIVE COVER over the rectangular, peach Clonidine Transdermal System PATCH and apply firm pressure, especially around the edges in contact with the skin.
Instructions for Disposal
KEEP OUT OF REACH OF CHILDREN
During or even after use, a PATCH contains active medication which may be harmful to infants and children if accidentally applied or ingested. After use, fold in half with the sticky sides together. Dispose of carefully out of reach of children.
Call your doctor for medical advice about side effects. You may report side effects to FDA at 1-800-FDA-1088.
For more information, call Mylan Pharmaceuticals Inc. at 1-877-446-3679 (1-877-4-INFO-RX).
Mylan Pharmaceuticals Inc. Morgantown, WV 26505 U.S.A.
REVISED JANUARY 2013 PL:CTS:R11
PRINCIPAL DISPLAY PANEL - 0.1 mg/day
Contents: 4 Systems and 4 Adhesive Covers
NDC 0378-0871-99
Rx only
Clonidine Transdermal System, USP
0.1 mg/day
Programmed delivery in vivo of 0.1 mg clonidine per day for one week.
Each system contains 2.52 mg of clonidine. The inactive components are mineral oil, polyisobutylene, colloidal silicon dioxide, pigmented polyethylene/polyester film, and silicone/polyester film.
FOR TRANSDERMAL USE ONLY.
Keep this and all drugs out of the reach of children.
See package insert for dosage information.
Store at 20° to 25°C (68° to 77°F). [See USP Controlled Room Temperature.]
Mylan Pharmaceuticals Inc. Morgantown, WV 26505 U.S.A.
Mylan.com
M0871-99-4C:R10
References
- ↑ "CLONIDINE PATCH [MYLAN PHARMACEUTICALS INC.]". Retrieved 5 February 2014.