Carbon-oxygen bond
A carbon-oxygen bond is a covalent bond between carbon and oxygen and one of the most abundant in organic chemistry and biochemistry [1]. Oxygen has 6 valence electrons and prefers to share two electrons in bonding with carbon, leaving the remaining 4 nonbonding in 2 lone pairs. The simplest representatives can be thought of organic derivatives of water: the alcohols.
A CO bond is strongly polarized towards oxygen (Electronegativity C vs O = 2.55:3.44) and many alcohols are water soluble. Bond lengths for paraffinic C-O bonds are in the range of 143 picometer shorter than that of C-N or C-C bonds. Shortened single bonds are found with carboxylic acids (136 pm) due to partial double bond character and elongated bonds are found in epoxides (147 pm) [2]. The CO bond strength is also larger compared to CN or CC for example that in methanol 91 kcal/mol (298 K) (87 in methyl amine and 88 in ethane)
Carbon and oxygen form terminal double bonds in functional groups collectively known as carbonyl compounds to which belong such compounds as ketones, esters and carboxylic acids and many more. Internal CO double bonds are found in positively charged Oxonium ions but mainly as reactive intermediates. In furans, the oxygen atom contributes to pi-electron delocalization via its filled p-orbital and hence furans are aromatic. Bond lengths of CO double bonds are around 123 pm in carbonyl compounds. The CO bond in acyl halides have a partial triple bond and subsequently very short: 117 pm. Compounds with formal CO triple bonds do not exist. The CO triple bond has also very high bond energy, even higher than NN triple bond[3].
Chemistry
Important carbon oxygen bond forming reactions are Williamson ether synthesis, nucleophilic acyl substitutions and electrophilic addition to alkenes. The Paterno-Buchi reaction is the carbonyl equivalent of of a metathesis reaction.
Oxygen functional groups
Carbon-oxygen bonds are present in these functional groups:
| Chemical class | Bond order | Formula | Structural Formula | Example |
|---|---|---|---|---|
| Alcohols | 1 | R3C-OH | 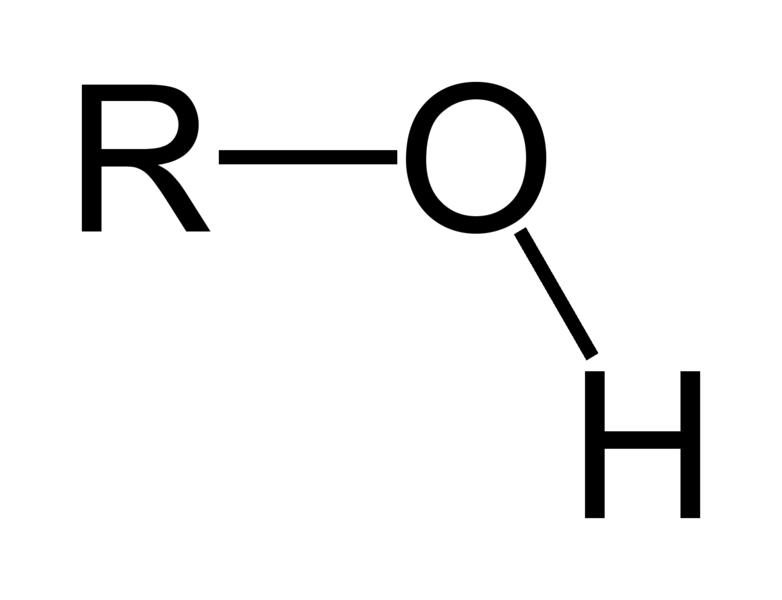
|
Ethanol |
| Ethers | 1 | R3C-O-CR3 | 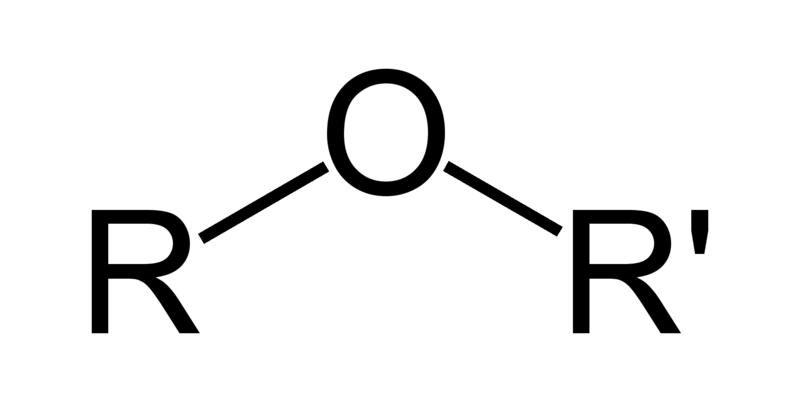
|
Diethyl ether |
| Peroxides | 1 | R3C-O-O-CR3 | 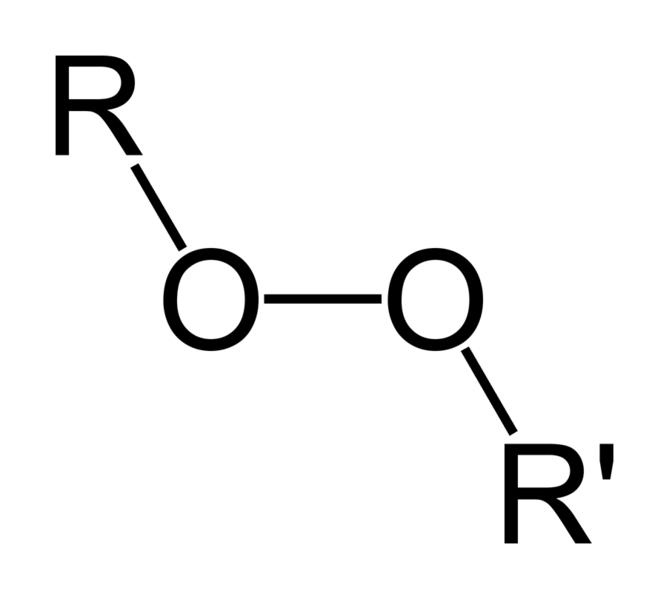
|
Di-tert-butyl peroxide |
| Esters | 1 | R3C-CO-O-CR3 | 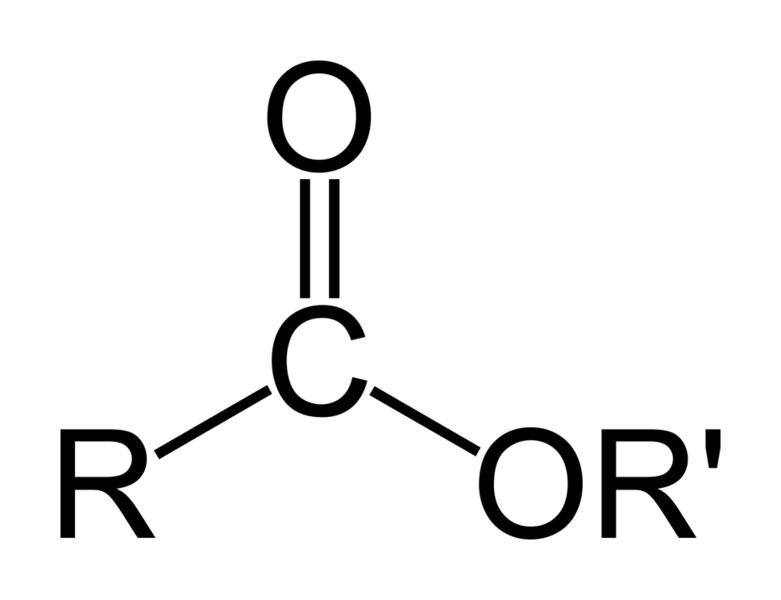
|
Ethyl acrylate Ethyl acrylate |
| Carbonate esters | 1 | R3C-O-CO-O-CR3 | Carbonate ester | Ethylene carbonate Ethylene carbonate |
| Ketones | 2 | R3C-CO-CR3 | 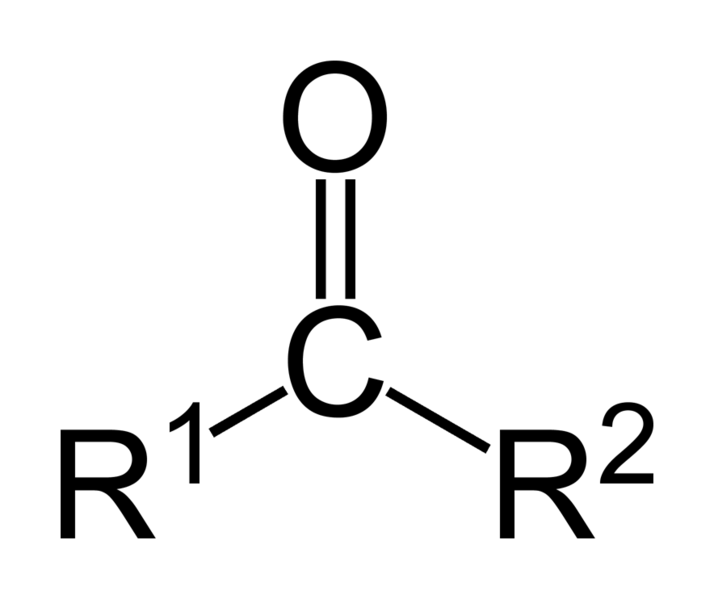
|
Acetone |
| Aldehydes | 2 | R3C-CHO | Aldehyde | Acrolein Acrolein |
| Furans | 1.5 | Furan | Furfurals Furfural | |
| Pyrylium salts | 1.5 | pyridine | Anthocyanins Anthocyanins |
See also
- The chemistry of carbon bonded to other elements in the periodic table:
Template:ChemicalBondsToCarbon
References
- ↑ Organic Chemistry John McMurry 2nd Ed.
- ↑ CRC Handbook of Chemistry and Physics 65Th Ed.
- ↑ Bond Energies