Base pair
Editor-In-Chief: C. Michael Gibson, M.S., M.D. [2]
In molecular biology, two nucleotides on opposite complementary DNA or RNA strands that are connected via hydrogen bonds are called a base pair (often abbreviated bp). In the canonical Watson-Crick base pairing, adenine (A) forms a base pair with thymine (T), as does guanine (G) with cytosine (C) in DNA. In RNA, thymine is replaced by uracil (U). Non-Watson-Crick base pairing with alternate hydrogen bonding patterns also occur, especially in RNA; common such patterns are Hoogsteen base pairs. Pairing is also the mechanism by which codons on messenger RNA molecules are recognized by anticodons on transfer RNA during protein translation. Some DNA- or RNA-binding enzymes can recognize specific base pairing patterns that identify particular regulatory regions of genes.
The size of an individual gene or an organism's entire genome is often measured in base pairs because DNA is usually double-stranded. Hence, the number of total base pairs is equal to the number of nucleotides in one of the strands (with the exception of non-coding single-stranded regions of telomeres). The haploid human genome (23 chromosomes) is estimated to be about 3 billion base pairs long and to contain 20,000-25,000 distinct genes.[1]
Examples
The following DNA sequences illustrate pair double-stranded patterns. By convention, the top strand is written from the 5' end to the 3' end; thus the bottom strand is written 3' to 5'.
- A base-paired DNA sequence:
ATCGAT TAGCTA
- The corresponding base-paired RNA sequence, in which uracil is substituted for thymine:
AUCGAU UAGCUA
Length measurements
The following abbreviations are commonly used to describe the length of a DNA/RNA molecule:
- bp = base pair(s)
- kb (= kbp) = kilo base pairs = 1,000 bp
- Mb = mega base pairs = 1,000,000 bp
- Gb = giga base pairs = 1,000,000,000 bp
In case of single stranded DNA/RNA we talk about nucleotides, abbreviated nt (or knt, Mnt, Gnt), rather than base pairs, as they are not paired. For distinction between units of computer storage and bases kbp, Mbp, Gbp etc may be used for disambiguation.
The Centimorgan is also often used to imply distance along a chromosome, but the number of base-pairs it corresponds to varies widely. In the Human genome, it is about 1 million base pairs[2] [3].
Hydrogen bonding and stability
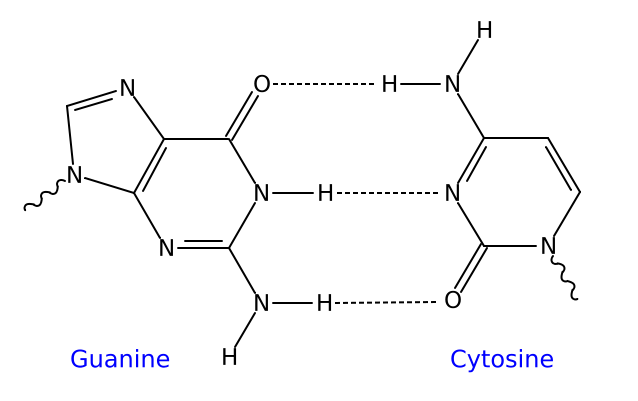
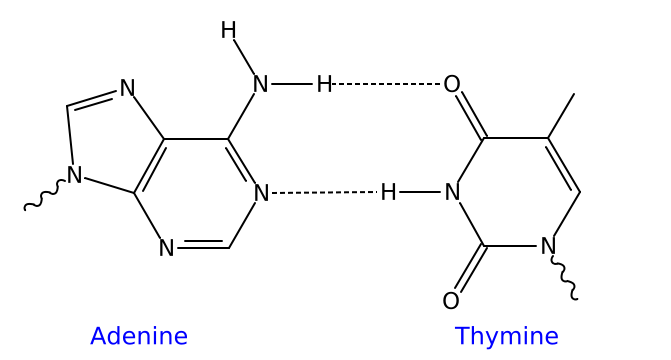
Hydrogen bonding is the chemical mechanism that underlies the base-pairing rules described above. Appropriate geometrical correspondence of hydrogen bond donors and acceptors allows only the "right" pairs to form stably. The GC base pair has three hydrogen bonds, whereas the AT base pair has only two; as a consequence, the GC pair is more stable.
The larger nucleic acids, adenine and guanine, are members of a class of doubly-ringed chemical structures called purines; the smaller nucleic acids, cytosine and thymine (and uracil), are members of a class of singly-ringed chemical structures called pyrimidines. Purines are only complementary with pyrimidines: pyrimidine-pyrimidine pairings are energetically unfavorable because the molecules are too far apart for hydrogen bonding to be established; purine-purine pairings are energetically unfavorable because the molecules are too close, leading to electrostatic repulsion. The only other possible pairings are GT and AC; these pairings are mismatches because the pattern of hydrogen donors and acceptors do not correspond. (It should be noted that the GU pairing, with two hydrogen bonds, does occur fairly often in RNA but rarely in DNA.)
Paired DNA and RNA molecules are comparatively stable at room temperature but the two nucleotide strands will separate above a melting point that is determined by the length of the molecules, the extent of mispairing (if any), and the GC content. Higher GC content results in higher melting temperatures; it is therefore unsurprising that the genomes of extremophile organisms such as Thermus thermophilus are particularly GC-rich. Conversely, regions of a genome that need to separate frequently - for example, the promoter regions for often-transcribed genes - are comparatively GC-poor (for example, see TATA box). GC content and melting temperature must also be taken into account when designing primers for PCR reactions.
Base stacking
Base stacking interactions between the pi orbitals of the bases' aromatic rings also contribute to stability, and again GC stacking interactions with adjacent bases tend to be more favorable. (Note, though, that a GC stacking interaction with the next base pair is geometrically different from a CG interaction.) Base stacking effects are especially important in the secondary structure of RNA; for example, RNA stem-loop structures are stabilized by base stacking in the loop region.
Base analogs and intercalators
Chemical analogs of nucleotides can take the place of proper nucleotides and establish non-canonical base-pairing, leading to errors (mostly point mutations) in DNA replication and DNA transcription. One common mutagenic base analog is 5-bromouracil, which resembles thymine but can base-pair to guanine in its enol form.
Other chemicals, known as DNA intercalators, fit into the gap between adjacent bases on a single strand and induce frameshift mutations by "masquerading" as a base, causing the DNA replication machinery to skip or insert additional nucleotides at the intercalated site. Most intercalators are large polyaromatic compounds and are known or suspected carcinogens. Examples include ethidium bromide and acridine.
See also
- DNA
- Nucleobase
- Wobble base pair
- Hoogsteen base pair
- List of binary polymorphisms
- Nucleic acid analogues
External links
- DAN - webserver version of the EMBOSS tool for calculating melting temperatures
Cited references
- ↑ International Human Genome Sequencing Consortium (2004). "Finishing the euchromatic sequence of the human genome". Nature. 431 (7011): 931–45. PMID 15496913. [1]
- ↑ The Genetic and Rare Diseases Information Center - Office of Rare Diseases redirect
- ↑ Matthew P Scott, Paul Matsudaira, Harvey Lodish, James Darnell, Lawrence Zipursky, Chris A Kaiser, Arnold Berk, Monty Krieger (2004). Molecular Cell Biology, Fifth Edition. San Francisco: W. H. Freeman. p. 396. ISBN 0-7167-4366-3.
...in humans 1 centimorgan on average represents a distance of about 7.5x10E5 base pairs
General references
- Watson JD, Baker TA, Bell SP, Gann A, Levine M, Losick R. (2004). Molecular Biology of the Gene. 5th ed. Pearson Benjamin Cummings: CSHL Press. See esp. ch. 6 and 9.
ca:Parell de bases de:Basenpaar id:Pasangan basa it:Coppia di basi hu:Bázispár nl:Basepaar simple:Base pair fi:Emäspari sv:Baspar uk:Пара основ