Apomorphine
Editor-In-Chief: C. Michael Gibson, M.S., M.D. [1]; Associate Editor(s)-in-Chief: Alberto Plate [2]
Disclaimer
WikiDoc MAKES NO GUARANTEE OF VALIDITY. WikiDoc is not a professional health care provider, nor is it a suitable replacement for a licensed healthcare provider. WikiDoc is intended to be an educational tool, not a tool for any form of healthcare delivery. The educational content on WikiDoc drug pages is based upon the FDA package insert, National Library of Medicine content and practice guidelines / consensus statements. WikiDoc does not promote the administration of any medication or device that is not consistent with its labeling. Please read our full disclaimer here.
Overview
Apomorphine is a non-ergoline dopamine agonist that is FDA approved for the treatment of advanced Parkinson's disease. Common adverse reactions include yawning, drowsiness/somnolence, dyskinesias, dizziness/postural hypotension, rhinorrhea, nausea and/or vomiting, hallucination/confusion, and edema/swelling of extremities.
Adult Indications and Dosage
FDA-Labeled Indications and Dosage (Adult)
Premedication and Concomitant Medication
- Apomorphine should be initiated with the use of a concomitant antiemetic. Oral trimethobenzamide (300 mg three times a day) should be started 3 days prior to the initial dose of Apomorphine and continued at least during the first two months of therapy. Based on reports of profound hypotension and loss of consciousness when apomorphine was administered with ondansetron, the concomitant use of apomorphine with drugs of the 5HT3 antagonist class including antiemetics (for example, ondansetron, granisetron, dolasetron, palonosetron) and alosetron are contraindicated.
Dosing Information
- The recommended starting dose of Apomorphine is 0.2 mL (2 mg). Titrate on the basis of effectiveness and tolerance, up to a maximum recommended dose of 0.6 mL (6 mg). There is no evidence from controlled trials that doses greater than 0.6 mL (6 mg) gave an increased effect and therefore, individual doses above 0.6 mL (6 mg) are not recommended. The average frequency of dosing in the development program was 3 times per day. There is limited experience with single doses greater than 0.6 mL (6 mg), dosing more than 5 times per day and with total daily doses greater than 2 mL (20 mg).
- Begin dosing when patients are in an "off" state. The initial dose should be a 0.2 mL (2 mg) test dose in a setting where medical personnel can closely monitor blood pressure and pulse. Both supine and standing blood pressure and pulse should be checked pre-dose and at 20 minutes, 40 minutes, and 60 minutes post-dose (and after 60 minutes, if there is significant hypotension at 60 minutes). Patients who develop clinically significant orthostatic hypotension in response to this test dose of Apomorphine should not be considered candidates for treatment with Apomorphine.
- If the patient tolerates the 0.2 mL (2 mg) dose, and responds adequately, the starting dose should be 0.2 mL (2 mg), used on an as needed basis to treat recurring "off" episodes. If needed, the dose can be increased in 0.1 mL (1 mg) increments every few days on an outpatient basis.
- The general principle guiding subsequent dosing (described in detail below) is to determine that the patient needs and can tolerate a higher test dose, 0.3 mL or 0.4 mL (3 mg or 4 mg, respectively) under close medical supervision. A trial of outpatient dosing may follow (periodically assessing both efficacy and tolerability), using a dose 0.1 mL (1 mg) lower than the tolerated test dose.
- If the patient tolerates the 0.2 mL (2 mg) test dose but does not respond adequately, a dose of 0.4 mL (4 mg) may be administered under medical supervision, at least 2-hours after the initial test dose, at the next observed "off" period. If the patient tolerates and responds to a test dose of 0.4 mL (4 mg), the initial maintenance dose should be 0.3 mL (3 mg) used on an as needed basis to treat recurring "off" episodes as an outpatient. If needed, the dose can be increased in 0.1 mL (1 mg) increments every few days on an outpatient basis.
- If the patient does not tolerate a test dose of 0.4 mL (4 mg), a test dose of 0.3 mL (3 mg) may be administered during a separate "off" period under medical supervision, at least 2-hours after the previous dose. If the patient tolerates the 0.3 mL (3 mg) test dose, the initial maintenance dose should be 0.2 mL (2 mg) used on an as needed basis to treat existing "off" episodes. If needed, and the 0.2 mL (2 mg) dose is tolerated, the dose can be increased to 0.3 mL (3 mg) after a few days. In such a patient, the dose should ordinarily not be increased to 0.4 mL (4 mg) on an out-patient basis.
Off-Label Use and Dosage (Adult)
Guideline-Supported Use
There is limited information regarding Off-Label Guideline-Supported Use of Apomorphine hydrochloride in adult patients.
Non–Guideline-Supported Use
Erectile Dysfunction
Restless Legs Syndrome
- Dosage: Subcutaneous injections of 12 to 18 milligrams/night[3].
Pediatric Indications and Dosage
FDA-Labeled Indications and Dosage (Pediatric)
There is limited information regarding Apomorphine FDA-Labeled Indications and Dosage (Pediatric) in the drug label.
Off-Label Use and Dosage (Pediatric)
Guideline-Supported Use
There is limited information regarding Off-Label Guideline-Supported Use of Apomorphine hydrochloride in pediatric patients.
Non–Guideline-Supported Use
There is limited information regarding Off-Label Non–Guideline-Supported Use of Apomorphine hydrochloride in pediatric patients.
Contraindications
Apomorphine is contraindicated in patients:
- Using concomitant drugs of the 5HT3 antagonist class including antiemetics (e.g., ondansetron, granisetron, dolasetron, palonosetron) and alosetron. There have been reports of profound hypotension and loss of consciousness when Apomorphine was administered with ondansetron.
- With hypersensitivity/allergic reaction characterized by urticaria, rash, pruritus, and/or various manifestations of angioedema to apomorphine or to any of the excipients including a sulfite (i.e., sodium metabisulfite). Patients with a sulfite sensitivity may experience various allergic-type reactions, including anaphylactic symptoms and life-threatening asthmatic attacks. Patients who experience any hypersensitivity/allergic reaction to Apomorphine should avoid taking Apomorphine again.
Warnings
Serious Adverse Reactions After Intravenous Administration
- Following intravenous administration of Apomorphine, serious adverse reactions including thrombus formation and pulmonary embolism due to intravenous crystallization of apomorphine have occurred. Consequently, Apomorphine should not be administered intravenously.
Nausea and Vomiting
- Apomorphine causes severe nausea and vomiting when it is administered at recommended doses. Because of this, in domestic clinical studies, 98% of all patients were pre-medicated with trimethobenzamide, an antiemetic, for three days prior to study enrollment, and were then encouraged to continue trimethobenzamide for at least 6 weeks. Even with the use of concomitant trimethobenzamide in clinical studies, 31% and 11% of the Apomorphine-treated patients had nausea and vomiting, respectively, and 3% and 2% of the patients discontinued Apomorphine due to nausea and vomiting, respectively. Among 522 patients treated, 262 (50%) discontinued trimethobenzamide while continuing Apomorphine The average time to discontinuation of trimethobenzamide was about 2 months (range: 1 day to 33 months). For the 262 patients who discontinued trimethobenzamide, 249 patients continued apomorphine without trimethobenzamide for a duration of follow-up that averaged 1 year (range: 0 years to 3 years).
- The ability of concomitantly administered antiemetic drugs (other than trimethobenzamide) to reduce the incidence of nausea and/or vomiting in Apomorphine-treated patients has not been studied. Antiemetics with anti-dopaminergic actions (e.g., haloperidol, chlorpromazine, promethazine, prochlorperazine, metaclopramide) have the potential to worsen the symptoms in patients with Parkinson's disease and should be avoided.
Falling Asleep During Activities of Daily Living and Somnolence
- There have been reports in the literature of patients treated with Apomorphine subcutaneous injections who suddenly fell asleep without prior warning of sleepiness while engaged in activities of daily living. Somnolence is commonly associated with Apomorphine and it is reported that falling asleep while engaged in activities of daily living always occurs in a setting of pre-existing somnolence, even if patients do not give such a history. Somnolence was reported in 35% of patients treated with Apomorphine and in none of the patients in the placebo group. Prescribers should reassess patients for drowsiness or sleepiness, especially since some of the events occur well after the start of treatment. Prescribers should also be aware that patients may not acknowledge drowsiness or sleepiness until directly questioned about drowsiness or sleepiness during specific activities.
- Before initiating treatment with Apomorphine, advise patients of the risk of drowsiness and ask them about factors that could increase the risk with Apomorphine, such as concomitant sedating medications and the presence of sleep disorders. If a patient develops significant daytime sleepiness or falls asleep during activities that require active participation (e.g., conversations, eating, etc.), Apomorphine should ordinarily be discontinued. If a decision is made to continue Apomorphine patients should be advised not to drive and to avoid other potentially dangerous activities. There is insufficient information to determine whether dose reduction will eliminate episodes of falling asleep while engaged in activities of daily living.
Syncope
- In clinical studies, approximately 2% of Apomorphine-treated patients experienced syncope.
Hypotension / Orthostatic Hypotension
- Dopamine agonists, including Apomorphine, may cause orthostatic hypotension at any time but especially during dose escalation. Patients with Parkinson's disease may also have an impaired capacity to respond to an orthostatic challenge. For these reasons, Parkinson's disease patients being treated with dopaminergic agonists ordinarily require careful monitoring for signs and symptoms of orthostatic hypotension, especially during dose escalation, and should be informed of this risk.
- Patients undergoing titration of Apomorphine showed an increased incidence (from 4% pre-dose to 18% post-dose) of systolic orthostatic hypotension (≥ 20 mmHg decrease) when evaluated at various times after in-office dosing. A small number of patients developed severe systolic orthostatic hypotension (≥ 30 mmHg decrease and systolic BP ≤ 90 mmHg) after subcutaneous apomorphine injection. In clinical trials of Apomorphine in patients with advanced Parkinson's disease, 59 of 550 patients (11%) had orthostatic hypotension, hypotension, and/or syncope. These events were considered serious in 4 patients (< 1%) and resulted in withdrawal of Apomorphine in 10 patients (2%). These events occurred both with initial dosing and during long-term treatment. Whether or not hypotension contributed to other significant adverse events seen (e.g., falls), is unknown. Apomorphine causes dose-related decreases in systolic blood pressure (SBP) and diastolic blood pressure (DBP).
- The hypotensive effects of Apomorphine may be increased by the concomitant use of alcohol, antihypertensive medications, and vasodilators (especially nitrates). Patients should avoid alcohol when using Apomorphine. Check blood pressure for hypotension and orthostatic hypotension in patients Apomorphine with concomitant antihypertensive medications and/or vasodilators.
Falls
- Patients with Parkinson's disease (PD) are at risk of falling due to underlying postural instability, possible autonomic instability, and syncope caused by the blood pressure lowering effects of the drugs used to treat PD. Subcutaneous Apomorphine might increase the risk of falling by simultaneously lowering blood pressure and altering mobility. In clinical trials, 30% of patients had events that could reasonably be considered falls and about 5% of patients had falls that were considered serious.
Hallucinations / Psychotic-Like Behavior
- In clinical studies, hallucinations were reported by 14% of the Apomorphine-treated patients. In one randomized, double-blind, placebo-controlled study, hallucinations or confusion occurred in 10% of patients treated with Apomorphine and 0% of patients treated with placebo. Hallucinations resulted in discontinuation of Apomorphine in 1% of patients.
- Post marketing reports indicate that patients may experience new or worsening mental status and behavioral changes, which may be severe, including psychotic-like behavior after starting or increasing the dose of Apomorphine. Other drugs prescribed to improve the symptoms of Parkinson's disease can have similar effects on thinking and behavior. This abnormal thinking and behavior can consist of one or more of a variety of manifestations, including paranoid ideation, delusions, hallucinations, confusion, disorientation, aggressive behavior, agitation, and delirium.
- Patients with a major psychotic disorder should ordinarily not be treated with Apomorphine because of the risk of exacerbating psychosis. In addition, certain medications used to treat psychosis may exacerbate the symptoms of Parkinson's disease and may decrease the effectiveness of Apomorphine.
Dyskinesias
- Apomorphine may cause dyskinesia or exacerbate pre-existing dyskinesia. In clinical studies, dyskinesia or worsening of dyskinesia was reported in 24% of patients. Overall, 2% of Apomorphine-treated patients withdrew from studies due to dyskinesias.
Impulse Control/Compulsive Behaviors
- Case reports suggest that patients can experience intense urges to gamble, increased sexual urges, intense urges to spend money uncontrollably, and other intense urges and the inability to control these urges while taking one or more of the medications, including Apomorphine, that increase central dopaminergic tone and that are generally used for the treatment of Parkinson's disease. In some cases, although not all, these urges were reported to have stopped when the dose was reduced or the medication was discontinued. Because patients may not recognize these behaviors as abnormal it is important for prescribers to specifically ask patients or their caregivers about the development of new or increased gambling urges, sexual urges, uncontrolled spending or other urges while being treated with Apomorphine Physicians should consider dose reduction or stopping the medication if a patient develops such urges while taking Apomorphine.
Coronary Events
- In clinical studies, 4% of patients treated with Apomorphine experienced angina, myocardial infarction, cardiac arrest and/or sudden death; some cases of angina and myocardial infarction occurred in close proximity to Apomorphine dosing (within 2 hours), while other cases of cardiac arrest and sudden death were observed at times unrelated to dosing. Apomorphine has been shown to reduce resting systolic and diastolic blood pressure and may have the potential to exacerbate coronary (and cerebral) ischemia in patients with known cardiovascular and cerebrovascular disease. If patients develop signs and symptoms of coronary or cerebral ischemia, prescribers should re-evaluate the continued use of Apomorphine.
QTc Prolongation and Potential for Proarrhythymic Effects
- There is a small dose related prolongation of QTc interval with doses of Apomorphine greater than 6 mg. Doses greater than 6 mg do not provide additional clinical benefit and are not recommended. Drugs that prolong the QTc interval have been associated with torsades de pointes and sudden death. The relationship of QTc prolongation to torsades de pointes is clearest for larger increases (20 msec and greater), but it is possible that smaller QTc prolongations may also increase risk, or increase it in susceptible individuals, such as those with hypokalemia, hypomagnesemia, bradycardia, concomitant use of other drugs that prolong the QTc interval, or genetic predisposition (e.g., congenital prolongation of the QT interval). Although torsades de pointes has not been observed in association with the use of Apomorphine at recommended doses in clinical studies, experience is too limited to rule out an increased risk. Palpitations and syncope may signal the occurrence of an episode of torsades de pointes. The risks and benefits of Apomorphine treatment should be considered prior to initiating treatment with Apomorphine in patients with risk factors for prolonged QTc.
Withdrawal-Emergent Hyperpyrexia and Confusion
- A symptom complex resembling the neuroleptic malignant syndrome (characterized by elevated temperature, muscular rigidity, altered consciousness, and autonomic instability), with no other obvious etiology, has been reported in association with rapid dose reduction, withdrawal of, or changes in antiparkinsonian therapy.
Melanoma
- Epidemiological studies have shown that patients with Parkinson's disease have a higher risk (2- to approximately 6-fold higher) of developing melanoma than the general population. Whether the increased risk observed was due to Parkinson's disease or other factors, such as drugs used to treat Parkinson's disease, is unclear. For the reasons stated above, patients and providers are advised to monitor for melanomas frequently and on a regular basis when using Apomorphine for any indication. Ideally, periodic skin examinations should be performed by appropriately qualified individuals (e.g., dermatologists).
Fibrotic Complications
- Cases of retroperitoneal fibrosis, pulmonary infiltrates, pleural effusion, pleural thickening, and cardiac valvulopathy have been reported in some patients treated with ergot-derived dopaminergic agents. While these complications may resolve when the drug is discontinued, complete resolution does not always occur. Although these adverse reactions are believed to be related to the ergoline structure of these dopamine agonists, whether other, nonergot derived dopamine agonists, such as Apomorphine can cause these reactions is unknown.
Priapism
- Apomorphine may cause prolonged painful erections in some patients. In clinical studies, painful erections were reported by 3 of 361 Apomorphine-treated men, and one patient withdrew from Apomorphine therapy because of priapism. Although no patients in the clinical studies required surgical intervention, severe priapism may require surgical intervention.
Retinal Pathology in Albino Rats
- In a 2-year carcinogenicity study of apomorphine in albino rat, retinal atrophy was detected at all subcutaneous doses tested (up to 0.8 mg/kg/day or 2 mg/kg/day in males or females, respectively; less than the maximum recommended human dose of 20 mg/day on a body surface area (mg/m2) basis). Retinal atrophy/degeneration has been observed in albino rats treated with other dopamine agonists for prolonged periods (generally during 2-year carcinogenicity studies). Retinal findings were not observed in a 39-week subcutaneous toxicity study of apomorphine in monkey at doses up to 1.5 mg/kg/day, a dose similar to the MRHD on a mg/m2 basis. The clinical significance of the finding in rat has not been established but cannot be disregarded because disruption of a mechanism that is universally present in vertebrates (e.g., disk shedding) may be involved.
Adverse Reactions
Clinical Trials Experience
Because clinical trials are conducted under widely varying conditions, the incidence of adverse reactions (number of unique patients experiencing an adverse reaction associated with treatment per total number of patients treated) observed in the clinical trials of a drug cannot be directly compared to the incidence of adverse reactions in the clinical trials of another drug and may not reflect the incidence of adverse reactions observed in practice.
- In placebo-controlled trials, most patients received only one subcutaneous dose of Apomorphine. All patients received concomitant levodopa and 86% received a concomitant dopamine agonist. All patients had some degree of spontaneously occurring periods of hypomobility ("off episodes") at baseline.
- The most common adverse reactions (Apomorphine incidence at least 10% greater than placebo incidence) observed in a placebo-controlled trial were yawning, drowsiness/somnolence, dyskinesias, dizziness/postural hypotension, rhinorrhea, nausea and/or vomiting, hallucination/confusion, and edema/swelling of extremities.
Table 1 presents the most common adverse reactions reported by Apomorphine-naïve Parkinson's disease patients who were enrolled in a randomized placebo-controlled, parallel group trial and who were treated for up to 4 weeks (Study 1). Individual Apomorphine doses in this trial ranged from 2 mg to 10 mg, and were titrated to achieve tolerability and control of symptoms.

Injection Site Reactions
- Patients treated with Apomorphine subcutaneous injections during clinical studies, 26% of patients had injection site reactions, including bruising (16%), granuloma (4%), and pruritus (2%). In addition to those in Table 1, the most common adverse reactions in pooled Apomorphine trials (occurring in at least 5% of the patients) in descending order were injection site reaction, fall, arthralgia, insomnia, headache, depression, urinary tract infection, anxiety, congestive heart failure, limb pain, back pain, Parkinson's disease aggravated, pneumonia, confusion, sweating increased, dyspnea, fatigue, ecchymosis, constipation, diarrhea, weakness, and dehydration.
Postmarketing Experience
There is limited information regarding Apomorphine Postmarketing Experience in the drug label.
Drug Interactions
5HT3 Antagonists
- Based on reports of profound hypotension and loss of consciousness when Apomorphine was administered with ondansetron, the concomitant use of Apomorphine with 5HT3 antagonists, including antiemetics (for example, ondansetron, granisetron, dolasetron, palonosetron) and alosetron, is contraindicated.
Antihypertensive Medications and Vasodilators
- The following adverse events were experienced more commonly in patients receiving concomitant antihypertensive medications or vasodilators (n = 94) compared to patients not receiving these concomitant drugs (n = 456): hypotension 10% vs 4%, myocardial infarction 3% vs 1%, serious pneumonia 5% vs 3%, serious falls 9% vs 3%, and bone and joint injuries 6% vs 2%. The mechanism underlying many of these events is unknown, but may represent increased hypotension.
Dopamine Antagonists
- Since Apomorphine is a dopamine agonist, it is possible that concomitant use of dopamine antagonists, such as the neuroleptics (phenothiazines, butyrophenones, thioxanthenes) or metoclopramide, may diminish the effectiveness of Apomorphine. Patients with major psychotic disorders, treated with neuroleptics, should be treated with dopamine agonists only if the potential benefits outweigh the risks.
Drugs Prolonging the QT/QTc Interval
- Caution should be exercised when prescribing Apomorphine concomitantly with drugs that prolong the QT/QTc interval.
Use in Specific Populations
Pregnancy
- There are no adequate and well-controlled studies in pregnant women. Apomorphine has been shown to be teratogenic in rabbits and embryolethal in rats when given at clinically relevant doses. Apomorphine should be used during pregnancy only if the potential benefit justifies the potential risk to the fetus.
- No adverse developmental effects were observed when apomorphine (0.3 mg/kg/day, 1 mg/kg/day, 3 mg/kg/day) was administered by subcutaneous injection to pregnant rat throughout organogenesis; the highest dose tested (3 mg/kg/day) is 1.5 times the MRHD (20 mg/day) on a mg/m2 basis. Administration of apomorphine (0.3 mg/kg/day, 1 mg/kg/day, 3 mg/kg/day) by subcutaneous injection to pregnant rabbits throughout organogenesis resulted in an increased incidence of malformations of the heart and/or great vessels at the mid and high doses tested; the no-effect dose is less than the MRHD on a mg/m2 basis.
- Apomorphine (0.3 mg/kg/day, 1 mg/kg/day, 3 mg/kg/day), administered by subcutaneous injection to females throughout gestation and lactation, resulted in increased offspring mortality at the highest dose tested. There were no effects on developmental parameters or reproductive performance in surviving offspring. The no-effect dose for developmental toxicity (1 mg/kg/day) is less than the MRHD on a mg/m2 basis.
Pregnancy Category (AUS):
There is no Australian Drug Evaluation Committee (ADEC) guidance on usage of Apomorphine in women who are pregnant.
Labor and Delivery
There is no FDA guidance on use of Apomorphine during labor and delivery.
Nursing Mothers
- It is not known whether this drug is excreted in human milk. Because many drugs are excreted in human milk and because of the potential for serious adverse reactions in nursing infants from Apomorphine, a decision should be made as to whether to discontinue nursing or to discontinue the drug, taking into account the importance of the drug to the mother.
Pediatric Use
- Safety and effectiveness in pediatric patients have not been established.
Geriatic Use
- In the Apomorphine clinical development program, there were 239 patients less than age 65 treated with Apomorphine and 311 patients who were age 65 years of age or older. Confusion and hallucinations were reported more frequently with patients age 65 and older compared to patients with less than age 65. Serious adverse reactions (life-threatening events or events resulting in hospitalization and/or increased disability) were also more common in patients age 65 and older. Patients age 65 and older were more likely to fall (experiencing bone and joint injuries), have cardiovascular events, develop respiratory disorders, and have gastrointestinal events. Patients age 65 and above were also more likely to discontinue Apomorphine treatment as a result of one or more adverse reactions.
Gender
There is no FDA guidance on the use of Apomorphine with respect to specific gender populations.
Race
There is no FDA guidance on the use of Apomorphine with respect to specific racial populations.
Renal Impairment
- The starting Apomorphine dose should be reduced in patients with mild or moderate renal impairment because the concentration and exposure (Cmax and AUC) are increased in these patients. Studies in subjects with severe renal impairment have not been conducted .
Hepatic Impairment
- Caution should be exercised when administrating Apomorphine to patients with mild and moderate hepatic impairment due to the increased Cmax and AUC in these patients. Studies of subjects with severe hepatic impairment have not been conducted.
Females of Reproductive Potential and Males
There is no FDA guidance on the use of Apomorphine in women of reproductive potentials and males.
Immunocompromised Patients
There is no FDA guidance one the use of Apomorphine in patients who are immunocompromised.
Administration and Monitoring
Administration
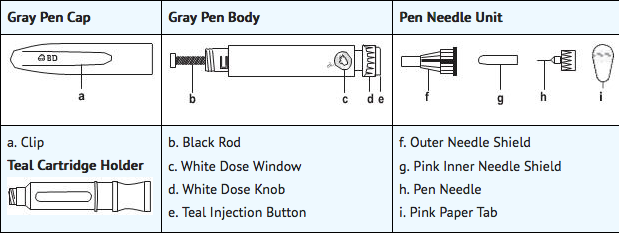
Apomorphine Pen Instructions of Administration

Monitoring
There is limited information regarding Apomorphine Monitoring in the drug label.
IV Compatibility
There is limited information regarding the compatibility of Apomorphine and IV administrations.
Overdosage
A 62-year-old man accidentally injected 25 mg of Apomorphine subcutaneously. After 3 minutes, the patient felt nauseated and lost consciousness for 20 minutes. Afterwards, he was alert with a heart rate 40/minute and a supine blood pressure of 90/50. He recovered completely within an hour.
Pharmacology
Mechanism of Action
- Apomorphine is a non-ergoline dopamine agonist with high in vitro binding affinity for the dopamine D4 receptor, and moderate affinity for the dopamine D2, D3, and D5, and adrenergic α1D, α2B, α2C receptors. The precise mechanism of action of Apomorphine as a treatment for Parkinson's disease is unknown, although it is believed to be due to stimulation of post-synaptic] dopamine D2-type receptors within the caudate-putamen in the brain.
Structure
- Apomorphine (apomorphine hydrochloride injection) contains apomorphine hydrochloride, a non-ergoline dopamine agonist. Apomorphine hydrochloride is chemically designated as 6aβ-Aporphine-10,11-diol hydrochloride hemihydrate with a molecular formula of C17H17NO2 ∙ HCL ∙ ½ H2O. Its structural formula and molecular weight are:

Pharmacodynamics
Prolongation of the QTc Interval
- In a placebo-controlled study in which patients received increasing single doses of Apomorphine from 2 mg to up to 10 mg, the mean difference in QTc (measured by Holter monitor) between Apomorphine and placebo was 0 msec at 4 mg, 1 msec at 6 mg, and 7 msec at 8 mg. Too few patients received a 10 mg dose to be able to adequately characterize the change in QTc interval at that dose.
- In a controlled trial in which patients were administered placebo or a single dose of Apomorphine (mean dose of 5.2 mg; range of 2 mg to 10 mg), the mean difference between Apomorphine and placebo in the change in QTc was about 3 msec at 20 minutes and 90 minutes. In the entire database, 2 patients (one at 2 mg and 6 mg, one at 6 mg) exhibited large QTc increments (> 60 msecs from pre-dose) and had QTc intervals greater than 500 msecs acutely after dosing. Doses of 6 mg or less thus are associated with minimal increases in QTc.
Decreases in blood pressure
Dose-dependent mean decrements in systolic blood pressure ranged from 5 mmHg after 2 mg to 16 mmHg after 10 mg. Dose-dependent mean decrements in diastolic blood pressure ranged from 3 mmHg after 2 mg to 8 mmHg after 10 mg. These changes were observed at 20 minutes, and were maximal between 20 and 40 minutes after dosing. Lesser, but still noteworthy blood pressure decrements persisted up to at least 90 minutes after dosing.
Pharmacokinetics
Absorption
- Apomorphine hydrochloride is a lipophilic compound that is rapidly absorbed (time to peak concentration ranges from 10 minutes to 60 minutes) following subcutaneous administration into the abdominal wall. After subcutaneous administration, apomorphine appears to have bioavailability equal to that of an intravenous administration. *Apomorphine exhibits linear pharmacokinetics over a dose range of 2 mg to 8 mg following a single subcutaneous injection of Apomorphine into the abdominal wall in patients with idiopathic Parkinson's disease.
Distribution
- The plasma-to-whole blood apomorphine concentration ratio is equal to one. Mean (range) apparent volume of distribution was 218 L (123 L to 404 L). Maximum concentrations in cerebrospinal fluid (CSF) are less than 10% of maximum plasma concentrations and occur 10 minutes to 20 minutes later.
Metabolism and Elimination
- The mean apparent clearance (range) is 223 L/hr (125 L/hr to 401 L/hr) and the mean terminal elimination half-life is about 40 minutes (range about 30 minutes to 60 minutes)he route of metabolism in humans is not known. Potential routes of metabolism in humans include sulfation, N-demethylation, glucuronidation and oxidation. In vitro, apomorphine undergoes rapid autooxidation.
Nonclinical Toxicology
Carcinogenesis
- Lifetime carcinogenicity studies of apomorphine were conducted in male (0.1 mg/kg/day, 0.3 mg/kg/day, 0.8 mg/kg/day) and female (0.3 mg/kg/day, 0.8 mg/kg/day, 2 mg/kg/day) rats. Apomorphine was administered by subcutaneous injection for 22 months or 23 months, respectively. In males, there was an increase in Leydig cell tumors at the highest dose tested, which is less than the MRHD (20 mg) on a mg/m2 basis. This finding is of questionable significance because the endocrine mechanisms believed to be involved in the production of Leydig cell tumors in rats are not relevant to humans. No drug-related tumors were observed in females; the highest dose tested is similar to the MRHD on a mg/m2 basis.
- In a 26-week carcinogenicity study in P53-knockout transgenic mice, there was no evidence of carcinogenic potential when apomorphine was administered by subcutaneous injection at doses up to 20 mg/kg/day (male) or 40 mg/kg/day (female).
Mutagenesis
- Apomorphine was mutagenic in the in vitro bacterial reverse mutation (Ames) and the in vitro mouse lymphoma tk assays. Apomorphine was clastogenic in the in vitro chromosomal aberration assay in human lymphocytes and in the in vitro mouse lymphoma tk assay. Apomorphine was negative in the in vivo micronucleus assay in mice.
Impairment of Fertility
- Apomorphine was administered subcutaneously at doses up to 3 mg/kg/day (approximately 1.5 times the MRHD on a mg/m2 basis) to male and female rats prior to and throughout the mating period and continuing in females through gestation day 6. There was no evidence of adverse effects on fertility or on early fetal viability. A significant decrease in testis weight was observed in a 39-week study in cynomolgus monkey at all subcutaneous dose tested (0.3 mg/kg/day, 1 mg/kg/day, 1.5 mg/kg/day); the lowest dose tested is less than the MRHD on a mg/m2 basis.
- In a published fertility study, apomorphine was administered to male rats at subcutaneous doses of 0.2 mg/kg, 0.8 mg/kg, and 2 mg/kg prior to and throughout the mating period. Fertility was reduced at the highest dose tested.
Clinical Studies
The effectiveness of Apomorphine in the acute symptomatic treatment of the recurring episodes of hypomobility, "off" episodes ("end-of-dose wearing off" and unpredictable "on/off" episodes), in patients with advanced Parkinson's disease was established in three randomized, controlled trials of Apomorphine given subcutaneously (Studies 1, 2, and 3). At baseline in these trials, the mean duration of Parkinson's disease was approximately 11 years. Whereas all patients were using concomitant L-dopa at baseline, 86% of patients were using a concomitant oral dopaminergic agonist, 31% were using a concomitant catechol-ortho-methyl transferase COMT) inhibitor, and 10% were using a concomitant monoamine B oxidase inhibitor. Study 1 was conducted in patients who did not have prior exposure to Apomorphine (i.e., Apomorphine naïve) and Studies 2 and 3 were conducted in patients with at least 3 months of Apomorphine use immediately prior to study enrollment. Almost all patients without prior exposure to Apomorphine began taking an antiemetic (trimethobenzamide) three days prior to starting Apomorphine and 50% of patients were able to discontinue the concomitant antiemetic, on average 2 months after initiating Apomorphine.
The change from baseline in Part III (Motor Examination) of the Unified Parkinson's Disease Rating Scale (UPDRS) served as the primary outcome assessment measure in each study. Part III of the UPDRS contains 14 items designed to assess the severity of the cardinal motor findings (e.g., tremor, rigidity, bradykinesia, postural instability, etc.) in patients with Parkinson's disease
Study Nº1
- Study 1 was a randomized, double-blind, placebo-controlled, parallel-group trial in 29 patients with advanced Parkinson's disease who had at least 2 hours of "off" time per day despite an optimized oral regimen for Parkinson's disease including levodopa and an oral dopaminergic agonist. Patients with atypical Parkinson's disease, psychosis, dementia, hypotension, or those taking dopamine antagonists were excluded from participation. In an office setting, hypomobility was allowed to occur by withholding the patients' Parkinson's disease medications overnight. The following morning, patients (in a hypomobile state) were started on study treatment in a 2:1 ratio (2 mg of Apomorphine or placebo given subcutaneously). At least 2 hours after the first dose, patients were given additional doses of study medication until they achieved a "therapeutic response" (defined as a response similar to the patient's response to their usual dose of levodopa) or until 10 mg of Apomorphine or placebo equivalent was given. At each injection re-dosing, the study drug dose was increased in 2 mg increments up to 4 mg, 6 mg, 8 mg, 10 mg of Apomorphine) or placebo equivalent.
Of the 20 patients randomized to Apomorphine 18 achieved a "therapeutic response" at about 20 minutes. The mean Apomorphine dose was 5.4 mg (3 patients on 2 mg, 7 patients on 4 mg, 5 patients on 6 mg, 3 patients on 8 mg, and 2 patients on 10 mg). In contrast, of the 9 placebo-treated patients, none reached a "therapeutic response." The mean change-from-baseline for UPDRS Part III score for Apomorphine group (highest dose) was statistically significant compared to that for the placebo group (Table 2).

Study Nº2
- Study 2 used a randomized, placebo-controlled crossover design of 17 patients with Parkinson's disease who had been using Apomorphine for at least 3 months. Patients received their usual morning doses of Parkinson's disease medications and were followed until hypomobility occurred, at which time they received either a single dose of subcutaneous Apomorphine (at their usual dose) and placebo on different days in random order. UPDRS Part III scores were evaluated over time. The mean dose of Apomorphine was 4 mg (2 patients on 2 mg, 9 patients on 3 mg, 2 patients on 4 mg, and 1 patient each on 4.5 mg, 5 mg, 8 mg, and 10 mg). The mean change-from-baseline UPDRS Part III score for the Apomorphine group was statistically significant compared to that for the placebo group (Table 3).

Study Nº3
- Study 3 used a randomized withdrawal design in 4 parallel groups from 62 patients (Apomorphine-35; Placebo-27) with Parkinson's disease who had been using Apomorphine for at least 3 months. Patients were randomized to one of the following 4 treatments dosed once by subcutaneous administration: Apomorphine at the usual dose (mean dose 4.6 mg), placebo at a volume matching the usual Apomorphine dose, Apomorphine at the usual dose + 2 mg (0.2 mL) (mean dose 5.8 mg), or placebo at a volume matching the usual Apomorphine dose + 0.2 mL. Patients received their usual morning doses of Parkinson's disease medications and were followed until hypomobility occurred, at which time they received the randomized treatment. Apomorphine doses ranged between 2 mg – 10 mg. The mean change-from-baseline for the Apomorphine group for UPDRS Part III scores at 20 minutes post dosing was statistically significant compared to that for the placebo group (Table 4). Figure 2 describes the mean change from baseline in UPDRS Motor Scores over time for pooled Apomorphine and placebo administration.


How Supplied
Apomorphine is supplied as a 10 mg/mL clear, colorless, sterile, solution in 3 mL (30 mg) glass cartridges.
- NDC 27505-004-05: Cartons of five 3 mL cartridges
- Apomorphine Pen: The pen injector is provided in a package with six needles and a carrying case.
Storage
Store at 25°C (77°F). Excursions permitted to 15°C to 30°C (59°F to 86°F)
Images
Drug Images
{{#ask: Page Name::Apomorphine |?Pill Name |?Drug Name |?Pill Ingred |?Pill Imprint |?Pill Dosage |?Pill Color |?Pill Shape |?Pill Size (mm) |?Pill Scoring |?NDC |?Drug Author |format=template |template=DrugPageImages |mainlabel=- |sort=Pill Name }}
Package and Label Display Panel

{{#ask: Label Page::Apomorphine |?Label Name |format=template |template=DrugLabelImages |mainlabel=- |sort=Label Page }}
Patient Counseling Information
There is limited information regarding Apomorphine Patient Counseling Information in the drug label.
Precautions with Alcohol
Alcohol-Apomorphine hydrochloride interaction has not been established. Talk to your doctor about the effects of taking alcohol with this medication.
Brand Names
Look-Alike Drug Names
There is limited information regarding Apomorphine Look-Alike Drug Names in the drug label.
Drug Shortage Status
Price
References
The contents of this FDA label are provided by the National Library of Medicine.
- ↑ Lal S, Tesfaye Y, Thavundayil JX, Thompson TR, Kiely ME, Nair NP; et al. (1989). "Apomorphine: clinical studies on erectile impotence and yawning". Prog Neuropsychopharmacol Biol Psychiatry. 13 (3–4): 329–39. PMID 2748870.
- ↑ Mirone VG, Stief CG (2001). "Efficacy of apomorphine SL in erectile dysfunction". BJU Int. 88 Suppl 3: 25–9. PMID 11578276.
- ↑ Reuter I, Ellis CM, Ray Chaudhuri K (1999). "Nocturnal subcutaneous apomorphine infusion in Parkinson's disease and restless legs syndrome". Acta Neurol Scand. 100 (3): 163–7. PMID 10478579.
{{#subobject:
|Label Page=Apomorphine |Label Name=Apok Package.png
}}