Amphotericin B
Editor-In-Chief: C. Michael Gibson, M.S., M.D. [1]; Associate Editor(s)-in-Chief: Alberto Plate [2]
Disclaimer
WikiDoc MAKES NO GUARANTEE OF VALIDITY. WikiDoc is not a professional health care provider, nor is it a suitable replacement for a licensed healthcare provider. WikiDoc is intended to be an educational tool, not a tool for any form of healthcare delivery. The educational content on WikiDoc drug pages is based upon the FDA package insert, National Library of Medicine content and practice guidelines / consensus statements. WikiDoc does not promote the administration of any medication or device that is not consistent with its labeling. Please read our full disclaimer here.
Black Box Warning
|
WARNING
See full prescribing information for complete Boxed Warning.
|
Overview
Amphotericin B is a antifungal, anti-Infective Agent, antiprotozoal , Dermatological Agent and Polyene that is FDA approved for the treatment of invasive aspergillosis, cryptococcosis (torulosis), North American blastomycosis, systemic candidiasis, coccidioidomycosis, histoplasmosis, zygomycosis including mucormycosis due to susceptible species of the genera Absidia, Mucor and Rhizopus, and infections due to related susceptible species of Conidiobolus and Basidiobolus, and sporotrichosis. Amphotericin B may be useful in the treatment of American mucocutaneous leishmaniasis, but it is not the drug of choice as primary therapy.. There is a Black Box Warning for this drug as shown here. Common adverse reactions include hypotension, thrombophlebitis, injection site pain, diarrhea, indigestion, loss of appetite, nausea, vomiting, normocystic normochromic anemia, arthralgia, myalgia, headache, tachypnea, fever, malaise, and shivering.
The drug is available as conventional amphotericin B (Amphotericin B deoxycholate; AmB-d) or as lipid-based formulations. Available lipid-based formulations include Amphotericin B Lipid Complex (ABLC; Abelcet), Amphotericin B cholesteryl sulfate complex (Amphotericin B Colloidal Dispersion; ABCD; Amphotec), and Liposomal Amphotericin B (LAmB; AmBisome).
Adult Indications and Dosage
FDA-Labeled Indications and Dosage (Adult)
- Amphotericin B for Injection should be administered by slow intravenous infusion. Intravenous infusion should be given over a period of approximately 2 to 6 hours (depending on the dose) observing the usual precautions for intravenous therapy. The recommended concentration for intravenous infusion is 0.1 mg/mL (1mg/10mL). Since patient tolerance varies greatly, the dosage of amphotericin B must be individualized and adjusted according to the patient's clinical status (e.g., site and severity of infection, etiologic agent, cardio-renal function, etc.). A single intravenous test dose (1 mg in 20 mL of 5% dextrose solution) administered over 20 to 30 minutes may be preferred. The patient's temperature, pulse, respiration, and blood pressure should be recorded every 30 minutes for 2 to 4 hours.
- In patients with good cardio-renal function and a well tolerated test dose, therapy is usually initiated with a daily dose of 0.25 mg/kg of body weight. However, in those patients having severe and rapidly progressive fungal infection, therapy may be initiated with a daily dose of 0.3 mg/kg of body weight. In patients with impaired cardio-renal function or a severe reaction to the test dose, therapy should be initiated with smaller daily doses (i.e., 5 to 10 mg). Depending on the patient's cardio-renal status, doses may gradually be increased by 5 to 10 mg per day to final daily dosage of 0.5 to 0.7 mg/kg.
- There are insufficient data presently available to define total dosage requirements and duration of treatment necessary for eradication of specific mycoses. The optimal dose is unknown. Total daily dosage may range up to 1.0 mg/kg per day or up to 1.5 mg/kg when given on alternate days.
- Sporotrichosis: Therapy with intravenous amphotericin B for sporotrichosis has ranged up to nine months with a total dose up to 2.5 g.
- Aspergillosis: Aspergillosis has been treated with amphotericin B intravenously for a period up to 11 months with a total dose up to 3.6 g.
- Rhinocerebral phycomycosis: This fulminating disease generally occurs in association with diabetic ketoacidosis. It is, therefore, imperative that diabetic control be restored in order for treatment with Amphotericin B for Injection to be successful. In contradistinction, pulmonary phycomycosis, which is more common in association with hematologic malignancies, is often an incidental finding at autopsy. A cumulative dose of at least 3 g of amphotericin B is recommended to treat rhinocerebral phycomycosis. Although a total dose of 3 to 4 g will infrequently cause lasting renal impairment, this would seem a reasonable minimum where there is clinical evidence of invasion of deep tissue. Since rhinocerebral phycomycosis usually follows a rapidly fatal course, the therapeutic approach must necessarily be more aggressive than that used in more indolent mycoses.
Off-Label Use and Dosage (Adult)
Guideline-Supported Use
Systemic Aspergillosis with HIV Coinfection
- Dosage [1]
- Amphotericin B deoxycholate 1 mg/kg/day IV
- Amphotericin B lipid formulation 5 mg/kg/day IV
Esophagus Candidiasis
- Dosage: 0.3–0.7 mg/kg daily IV [2]
Treatment and Prophylaxis of Coccidioidomycosis-HIV Coinfection
- Dosage[3]:
- Amphotericin B deoxycholate 0.7–1.0 mg/kg IV daily
- Lipid formulation amphotericin B 4–6 mg/kg IV daily
Community Acquired Pneumonia
- Antibiotic Spectrum[4]
Treatment of HIV-Cryptoccocosis Coinfection
- Dosage [5]
- Amphotericin B lipid complex 5 mg/kg IV daily plus flucytosine 25 mg/kg orally 4 times daily
- Amphotericin B deoxycholate 0.7 to 1 mg/kg IV daily plus flucytosine* 25 mg/kg orally 4 times daily
Candidemia un Non-Neutropenic Patients
- Dosage [6]
- Amphotericin B deoxycholate: 0.5–1.0 mg/kg daily IV
- Amphotericin B Lipid Formulation: 3–5 mg/kg daily IV
Febrile Neutropenia[7]
Renal Candidiasis
- Dosage: 0.3–0.6 mg/kg daily for 1–7 id resistent to Fluconazol[8]
Non–Guideline-Supported Use
Primary Amebic Meningoencephalitis
- Dosage: One single intravenous dose of 75 mg
Infection due to Penicillium marneffei
- Dosage: 0.6 milligram/kilogram/day IV + 400 mg/day Itraconazol PO[9]
Pediatric Indications and Dosage
FDA-Labeled Indications and Dosage (Pediatric)
There is limited information regarding FDA-Labeled Use of Amphotericin B in pediatric patients.
Off-Label Use and Dosage (Pediatric)
Guideline-Supported Use
Candidiasis in HIV Co-Infected Patients[10]
Candidemia un Non-Neutropenic Patients
- Dosage [11]
- Amphotericin B deoxycholate: 0.5–1.0 mg/kg daily IV
- Amphotericin B Lipid Formulation: 3–5 mg/kg daily IV
Non–Guideline-Supported Use
Neonatal Candidiasis
- Dosage: 3–5 mg/kg daily IV if no urinary tract involvement[12]
Contraindications
- This product is contraindicated in those patients who have shown hypersensitivity to amphotericin B or any other component in the formulation unless, in the opinion of the physician, the condition requiring treatment is life-threatening and amenable only to amphotericin B therapy.
Warnings
|
WARNING
See full prescribing information for complete Boxed Warning.
|
- Amphotericin B is frequently the only effective treatment available for potentially life-threatening fungal disease. In each case, its possible life-saving benefit must be balanced against its untoward and dangerous side effects. EXERCISE CAUTION to prevent inadvertent Amphotericin B overdose, which may result in potentially fatal cardiac or cardiopulmonary arrest. Verify the product name and dosage especially if dose exceeds 1.5 mg/kg.
Adverse Reactions
Clinical Trials Experience
Although some patients may tolerate full intravenous doses of amphotericin B without difficulty, most will exhibit some intolerance, often at less than the full therapeutic dose. Tolerance may be improved by treatment with aspirin, antipyretics (e.g., acetaminophen), antihistamines, or antiemetics. Meperidine (25 to 50 mg IV) has been shown in some patients to decrease the duration of shaking chills and fever that may accompany the infusion of amphotericin B. Administration of amphotericin B on alternate days may decrease anorexia and phlebitis.
Intravenous administration of small doses of adrenal corticosteroids just prior to or during the Amphotericin B infusion may help decrease febrile reactions. Dosage and duration of such corticosteroid therapy should be kept to a minimum.
Addition of heparin (1000 units per infusion), and the use of a pediatric scalp-vein needle may lessen the incidence of thrombophlebitis. Extravasation may cause chemical irritation.
The adverse reactions most commonly observed are:
Body as a Whole
- Fever (sometimes accompanied by shaking chills usually occurring within 15 to 20 minutes after initiation of treatment)
- Malaise
- Weight loss
Cardiopulmonary
Gastrointestinal
Hematologic
- Normochromic, normocytic anemia.
Local
- Pain at the injection site with or without phlebitis or thrombophlebitis.
Musculoskeletal
- Generalized pain, including muscle and joint pains.
Neurologic
Renal
- Decreased renal function and renal function abnormalities including
- Azotemia
- Hypokalemia
- Hyposthenuria
- Renal tubular acidosis
- Nephrocalcinosis. These usually improve with interruption of therapy. However, some permanent impairment often occurs, especially in those patients receiving large amounts (over 5 g) of amphotericin B or receiving other nephrotoxic agents.
- In some patients hydration and sodium repletion prior to amphotericin B administration may reduce the risk of developing nephrotoxicity. Supplemental alkali medication may decrease renal tubular acidosis.
The following adverse reactions have also been reported:
General (body as a whole)
Allergic
- Anaphylactoid and other allergic reactions
- Bronchospasm
- Wheezing
Cardiopulmonary
- Cardiac arrest
- Shock
- Cardiac failure
- Pulmonary edema
- Hypersensitivity pneumonitis
- Arrhythmias, including ventricular fibrillation
- Dyspnea
- Hypertension
Gastrointestinal
Hematologic
Neurologic
- Convulsions
- Hearing loss
- Tinnitus
- Transient vertigo
- Visual impairment
- Diplopia
- Peripheral neuropathy
- Encephalopathy
Renal
Postmarketing Experience
Dermatologic
- Rash, in particular maculopapular
- Pruritus
- Skin exfoliation
- Toxic epidermal necrolysis
- Stevens-Johnson syndrome
Renal
Drug Interactions
When administered concurrently, the following drugs may interact with amphotericin B:
- Antineoplastic agents: may enhance the potential for renal toxicity, bronchospasm and hypotension. Antineoplastic agents (e.g., nitrogen mustard, etc.) should be given concomitantly only with great caution.
- Corticosteroids and Corticotropin (ACTH): may potentiate amphotericin B-induced hypokalemia which may predispose the patient to cardiac dysfunction. Avoid concomitant use unless necessary to control side effects of amphotericin B. If used concomitantly, closely monitor serum electrolytes and cardiac function.
- Digitalis glycosides: amphotericin B-induced hypokalemia may potentiate digitalis toxicity. Serum potassium levels and cardiac function should be closely monitored and any deficit promptly corrected.
- Flucytosine: while a synergistic relationship with amphotericin B has been reported, concomitant use may increase the toxicity of flucytosine by possibly increasing its cellular uptake and/or impairing its renal excretion.
- Imidazoles (e.g., ketoconazole, miconazole, clotrimazole, fluconazole, etc.): in vitro and animal studies with the combination of amphotericin B and imidazoles suggest that imidazoles may induce fungal resistance to amphotericin B. Combination therapy should be administered with caution, especially in immnocompromised patients.
- Other nephrotoxic medications: agents such as aminoglycosides, cyclosporine, and pentamidine may enhance the potential for drug-induced renal toxicity, and should be used concomitantly only with great caution. Intensive monitoring of renal function is recommended in patients requiring any combination of nephrotoxic medications.
- Skeletal muscle relaxants: amphotericin B-induced hypokalemia may enhance the curariform effect of skeletal muscle relaxants (e.g., tubocurarine). Serum potassium levels should be monitored and deficiencies corrected.
- Leukocyte transfusions: acute pulmonary toxicity has been reported in patients receiving intravenous amphotericin B and leukocyte transfusions.
Use in Specific Populations
Pregnancy
- Reproduction studies in animals have revealed no evidence of harm to the fetus due to amphotericin B for injection. Systemic fungal infections have been successfully treated in pregnant women with amphotericin B for injection without obvious effects to the fetus, but the number of cases reported has been small. Because animal reproduction studies are not always predictive of human response, and adequate and well-controlled studies have not been conducted in pregnant women, this drug should be used during pregnancy only if clearly indicated.
- Australian Drug Evaluation Committee (ADEC) Pregnancy Category
There is no Australian Drug Evaluation Committee (ADEC) guidance on usage of Amphotericin B in women who are pregnant.
Labor and Delivery
There is no FDA guidance on use of Amphotericin B during labor and delivery.
Nursing Mothers
- It is not known whether amphotericin B is excreted in human milk. Because many drugs are excreted in human milk and considering the potential toxicity of amphotericin B, it is prudent to advise a nursing mother to discontinue nursing
Pediatric Use
- Safety and effectiveness in pediatric patients have not been established through adequate and well-controlled studies. Systemic fungal infections have been successfully treated in pediatric patients without reports of unusual side effects. Amphotericin B for Injection when administered to pediatric patients should be limited to the smallest dose compatible with an effective therapeutic regimen.
Geriatic Use
There is no FDA guidance on the use of Amphotericin B with respect to geriatric patients.
Gender
There is no FDA guidance on the use of Amphotericin B with respect to specific gender populations.
Race
There is no FDA guidance on the use of Amphotericin B with respect to specific racial populations.
Renal Impairment
There is no FDA guidance on the use of Amphotericin B in patients with renal impairment.
Hepatic Impairment
There is no FDA guidance on the use of Amphotericin B in patients with hepatic impairment.
Females of Reproductive Potential and Males
There is no FDA guidance on the use of Amphotericin B in women of reproductive potentials and males.
Immunocompromised Patients
There is no FDA guidance one the use of Amphotericin B in patients who are immunocompromised.
Administration and Monitoring
Administration
- Oral
- Intravenous
Monitoring
Renal function should be monitored frequently during amphotericin B therapy. It is also advisable to monitor on a regular basis liver function, serum electrolytes (particularly magnesium and potassium, blood counts, and hemoglobin concentrations. Laboratory test results should be used as a guide to subsequent dosage adjustments.
IV Compatibility
There is limited information regarding IV Compatibility of Amphotericin B in the drug label.
Overdosage
Amphotericin B overdoses can result in potentially fatal cardiac or cardiopulmonary arrest. If an overdose is suspected, discontinue therapy and monitor the patient's clinical status (e.g., cardiorespiratory, renal, and liver function, hematologic status, serum electrolytes) and administer supportive therapy, as required. Amphotericin B is not hemodialyzable. Prior to reinstituting therapy, the patient's condition should be stabilized (including correction of electrolyte deficiencies, etc.).
Pharmacology
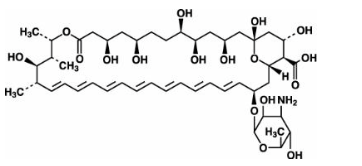
| |
Amphotericin B
| |
| Systematic (IUPAC) name | |
| (1R,3S,5R,6R,9R, 11R,15S,16R,17R,18S,19E,21E, 23E,25E,27E,29E,31E,33R,35S,36R,37S)- 33-[(3-amino- 3,6-dideoxy- β-D-mannopyranosyl)oxy]- 1,3,5,6,9,11,17,37-octahydroxy- 15,16,18-trimethyl- 13-oxo- 14,39-dioxabicyclo [33.3.1] nonatriaconta- 19,21,23,25,27,29,31-heptaene- 36-carboxylic acid | |
| Identifiers | |
| CAS number | |
| ATC code | A01 A07AA07 (WHO), G01AA03 (WHO), J02AA01 (WHO) |
| PubChem | |
| DrugBank | |
| Chemical data | |
| Formula | Template:OrganicBox atomTemplate:OrganicBox atomTemplate:OrganicBoxTemplate:OrganicBoxTemplate:OrganicBoxTemplate:OrganicBoxTemplate:OrganicBoxTemplate:OrganicBoxTemplate:OrganicBoxTemplate:OrganicBoxTemplate:OrganicBoxTemplate:OrganicBoxTemplate:OrganicBoxTemplate:OrganicBox atomTemplate:OrganicBoxTemplate:OrganicBox atomTemplate:OrganicBoxTemplate:OrganicBoxTemplate:OrganicBoxTemplate:OrganicBoxTemplate:OrganicBoxTemplate:OrganicBoxTemplate:OrganicBox |
| Mol. mass | 923.49 |
| SMILES | & |
| Pharmacokinetic data | |
| Bioavailability | 100% (IV) |
| Metabolism | renal |
| Half life | initial phase : 24 hours, second phase : approx. 15 days |
| Excretion | 40% found in urine after single cumulated over several days biliar excretion also important |
| Therapeutic considerations | |
| Pregnancy cat. |
B(US) |
| Legal status |
Rx-only, hospitalization recommended. |
| Routes | I.V. (slow infusion only) topical |
Mechanism of Action
- Amphotericin B is fungistatic or fungicidal depending on the concentration obtained in body fluids and the susceptibility of the fungus. The drug acts by binding to sterols in the cell membrane of susceptible fungi with a resultant change in membrane permeability allowing leakage of intracellular components. Mammalian cell membranes also contain sterols and it has been suggested that the damage to human cells and fungal cells may share common mechanisms.
Structure
- Amphotericin B is designated chemically as [1R- (1R*, 3S*, 5R*, 6R*, 9R*, 11R*, 15S*, 16R*, 17R*, 18S*, 19E, 21E, 23E, 25E, 27E, 29E, 31E, 33R*, 35S*, 36R*, 37S*)] -33-[(3-Amino-3, 6-dideoxy-β-D-mannopyranosyl)-oxy]-1,3,5,6,9,11,17,37-octahydroxy-15,16,18-trimethyl-13-oxo-14,39-dioxabicyclo [33.3.1] nonatriaconta-19,21,23,25,27,29,31-heptaene-36-carboxylic acid. Structural formula:

Pharmacodynamics
There is limited information regarding Pharmacodynamics of Amphotericin B in the drug label.
Pharmacokinetics
- An initial intravenous infusion of 1 to 5 mg of amphotericin B per day, gradually increased to 0.4 to 0.6 mg/kg daily, produces peak plasma concentrations ranging from approximately 0.5 to 2 mcg/mL. Following a rapid initial fall, plasma concentrations plateau at about 0.5 mcg/mL. An elimination half-life of approximately 15 days follows an initial plasma half-life of about 24 hours. Amphotericin B circulating in plasma is highly bound (>90%) to plasma proteins and is poorly dialyzable. Approximately two thirds of concurrent plasma concentrations have been detected in fluids from inflamed pleura, peritoneum, synovium, and aqueous humor. Concentrations in the cerebrospinal fluid seldom exceed 2.5% of those in the plasma. Little amphotericin B penetrates into vitreous humor or normal amniotic fluid. Complete details of tissue distribution are not known.
Amphotericin B is excreted very slowly (over weeks to months) by the kidneys with 2 to 5% of a given dose being excreted in the biologically active form. Details of possible metabolic pathways are not known. After treatment is discontinued, the drug can be detected in the urine for at least 7 weeks due to the slow disappearance of the drug. The cumulative urinary output over a 7 day period amounts to approximately 40% of the amount of drug infused.
Nonclinical Toxicology
There is limited information regarding Nonclinical Toxicology of Amphotericin B in the drug label.
Clinical Studies
There is limited information regarding Clinical Studies of Amphotericin B in the drug label.
How Supplied
- Available as single vials providing 50 mg amphotericin B as a yellow to orange lyophilized cake (which may partially reduce to powder following manufacture). NDC 39822-1055-5. Retain in carton until time of use.
Storage
- Prior to reconstitution Amphotericin B for Injection USP should be stored under refrigeration, 2˚ to 8˚C (36˚to 46˚F), protected against exposure to light. The concentrate (5 mg amphotericin B per mL after reconstitution with 10 mL Sterile Water for Injection USP) may be stored in the dark, at room temperature for 24 hours, or at refrigerator temperatures for one week with minimal loss of potency and clarity. Any unused material should then be discarded. Solutions prepared for intravenous infusion (0.1 mg or less amphotericin B per mL) should be used promptly after preparation and should be protected from light during administration.
Images
Drug Images
{{#ask: Page Name::Amphotericin B |?Pill Name |?Drug Name |?Pill Ingred |?Pill Imprint |?Pill Dosage |?Pill Color |?Pill Shape |?Pill Size (mm) |?Pill Scoring |?NDC |?Drug Author |format=template |template=DrugPageImages |mainlabel=- |sort=Pill Name }}
Package and Label Display Panel

{{#ask: Label Page::Amphotericin B |?Label Name |format=template |template=DrugLabelImages |mainlabel=- |sort=Label Page }}
Patient Counseling Information
There is limited information regarding Patient Counseling Information of Amphotericin B in the drug label.
Precautions with Alcohol
Alcohol-Amphotericin B interaction has not been established. Talk to your doctor about the effects of taking alcohol with this medication.
Brand Names
Look-Alike Drug Names
There is limited information regarding Amphotericin B Look-Alike Drug Names in the drug label.
Drug Shortage Status
Price
References
The contents of this FDA label are provided by the National Library of Medicine.
- ↑ "Guidelines for Prevention and Treatment of Opportunistic Infections in HIV-Infected Adults and Adolescents" (PDF).
- ↑ "Clinical Practice Guidelines for the Management of Candidiasis: 2009 Update by the Infectious Diseases Society of America" (PDF).
- ↑ "Guidelines for Prevention and Treatment of Opportunistic Infections in HIV-Infected Adults and Adolescents" (PDF).
- ↑ "Treatment of Community-Acquired Pneumonia".
- ↑ "Prevention and Treatment of Cryptococcosis Infection in HIV-Infected Persons".
- ↑ [Clinical Practice Guidelines for the Management of Candidiasis: 2009 Update by the Infectious Diseases Society of America Clinical Practice Guidelines for the Management of Candidiasis: 2009 Update by the Infectious Diseases Society of America] Check
|url=value (help). Missing or empty|title=(help) - ↑ "Clinical Practice Guideline for the Use of Antimicrobial Agents in Neutropenic Patients with Cancer: 2010 Update by the Infectious Diseases Society of America" (PDF).
- ↑ "Clinical Practice Guidelines for the Management of Candidiasis: 2009 Update by the Infectious Diseases Society of America".
- ↑ Sirisanthana T, Supparatpinyo K, Perriens J, Nelson KE (1998). "Amphotericin B and itraconazole for treatment of disseminated Penicillium marneffei infection in human immunodeficiency virus-infected patients". Clin Infect Dis. 26 (5): 1107–10. PMID 9597237.
- ↑ Mofenson LM, Brady MT, Danner SP, Dominguez KL, Hazra R, Handelsman E; et al. (2009). "Guidelines for the Prevention and Treatment of Opportunistic Infections among HIV-exposed and HIV-infected children: recommendations from CDC, the National Institutes of Health, the HIV Medicine Association of the Infectious Diseases Society of America, the Pediatric Infectious Diseases Society, and the American Academy of Pediatrics". MMWR Recomm Rep. 58 (RR-11): 1–166. PMC 2821196. PMID 19730409.
- ↑ [Clinical Practice Guidelines for the Management of Candidiasis: 2009 Update by the Infectious Diseases Society of America Clinical Practice Guidelines for the Management of Candidiasis: 2009 Update by the Infectious Diseases Society of America] Check
|url=value (help). Missing or empty|title=(help) - ↑ "Clinical Practice Guidelines for the Management of Candidiasis: 2009 Update by the Infectious Diseases Society of America".
{{#subobject:
|Label Page=Amphotericin B |Label Name=Amphotericin PL1.png
}}
{{#subobject:
|Label Page=Amphotericin B |Label Name=Amphotericin PL2.png
}}