Zwitterion
|
WikiDoc Resources for Zwitterion |
|
Articles |
|---|
|
Most recent articles on Zwitterion |
|
Media |
|
Evidence Based Medicine |
|
Clinical Trials |
|
Ongoing Trials on Zwitterion at Clinical Trials.gov Clinical Trials on Zwitterion at Google
|
|
Guidelines / Policies / Govt |
|
US National Guidelines Clearinghouse on Zwitterion
|
|
Books |
|
News |
|
Commentary |
|
Definitions |
|
Patient Resources / Community |
|
Patient resources on Zwitterion Discussion groups on Zwitterion Patient Handouts on Zwitterion Directions to Hospitals Treating Zwitterion Risk calculators and risk factors for Zwitterion
|
|
Healthcare Provider Resources |
|
Causes & Risk Factors for Zwitterion |
|
Continuing Medical Education (CME) |
|
International |
|
|
|
Business |
|
Experimental / Informatics |
Overview
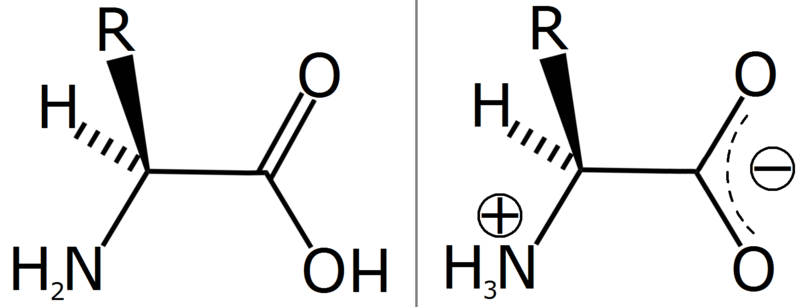
A zwitterion (from German "Zwitter" — "hybrid," "hermaphrodite") is a chemical compound that is electrically neutral but carries formal positive and negative charges on different atoms.[1] Zwitterions are polar and usually have a high solubility in water and a poor solubility in most organic solvents.
Ampholytes are molecules that contain both acidic and basic groups (and are therefore amphoteric) and will exist as zwitterions at a certain pH. This pH is known as the molecule's isoelectric point. Ampholytic molecules make good buffer solutions — they resist change to the pH of a solution by selective ionization. In the presence of acids, they will accept the hydrogen ions, removing them from the solution. In the presence of bases, they will donate hydrogen ions to the solution, again balancing the pH.
Typical examples of zwitterions are:
- Used as buffering agents most of which are included in Good's buffers:
- The amino-sulfonic acid based MES, MOPS, HEPES, PIPES or CAPS
- The amino-carboxylic acid (amino acid) based glycine, its derivatives bicine and tricine, and alanine
- Used as detergents:
- Natural products like the alkaloids psilocybin and lysergic acid.
- Betaines
Less common examples of zwitterions are:
- Quinonoid zwitterions.
- Drugs such as Fexofenadine (Allegra®).
References