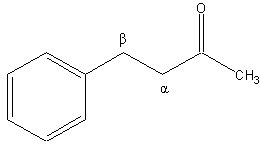
Alpha carbon
The alpha carbon in organic chemistry refers to the first carbon after the carbon that attaches to the functional group (the carbon is attached at the first, or alpha, position).[1] By extension, the second carbon is the "beta"-carbon,[2] and so on. This nomenclature can also be applied to the hydrogen atoms attached to the carbons. A hydrogen attached to an alpha carbon is called an "alpha-hydrogen" (α-hydrogen), a hydrogen on the beta-carbon is a beta-hydrogen, and so on.
This naming standard is sometimes considered to be not in compliance with IUPAC nomenclature (which encourages that carbons be identified by number, not by Greek letter); but it nonetheless remains very popular, particularly because it is useful in identifying the relative location of carbons to other functional groups (often a carbonyl).
α-carbon is also a term that applies to proteins and amino acids. It is the backbone carbon next to the carbonyl carbon. Therefore, reading along the backbone of a typical protein would give a sequence of carbonyl C, α-C, N, carbonyl C, α-C, N, and so on (when reading in the C to N direction). The α-carbon is where the different substituents attach to each different amino acid. That is, the groups hanging off the chain at the α-carbon are what give amino acids their diversity. These groups give the α-carbon its stereogenic properties for every amino acid except for glycine. Therefore, the α-carbon is a stereocenter for every amino acid except glycine.
The α-carbon of an amino acid is significant in protein folding. When describing a protein (which is a chain of amino acids), one often approximates the location of each amino acid as the location of its α-carbon. In general, α-carbons of adjacent amino acids in a protein are about 3.8 angstroms (380 picometers) apart.
The α-carbon is important for enol and enolate based carbonyl chemistry as well. Chemical transformations effected by the conversion to either an enolate or enol generally lead to the α-carbon acting as a nucleophile becoming, for example, alkyated in the presence of primary haloalkane. An exception is in reaction with silyl- chlorides, -bromides, and -iodides, where the oxygen acts as the nucleophile to produce silyl enol ether.
In ketones (a type of carbonyl) with acidic alpha hydrogen atoms on either side of the carbonyl carbon, selectivity of deprotonation may be achieved under select conditions. At low temperatures (-78°C, i.e. dry ice bath), in aprotic solvents, and with bulky non-equilibrating bases (e.g. LDA) the "kinetic" proton may be removed. The "kinetic" proton is the one which is sterically most accessible. Under thermodynamic conditions (warmer temperatures, weak base, and protic solvent) equilibrium is established between the ketone and the two possible enolates, the enolate favoured is termed the "thermodynamic" enolate and is favoured because of its lower energy level than the other possible enolate. Thus, by choosing the "correct" conditions to generate an enolate one can increase the yield of the desired product and minimize the formation of the undesired product.