Acute flaccid myelitis
Editor-In-Chief: C. Michael Gibson, M.S., M.D. [1]; Associate Editor(s)-in-Chief: Syed Hassan A. Kazmi BSc, MD [2]
Synonyms and keywords:; Acute flaccid paralysis, acute flaccid paralysis with anterior myelitis, paralysis with anterior myelitis, AFM, Enterovirus D68-Associated Anterior Myelitis
Overview
Acute flaccid myelitis (AFM) is a rare but serious neurological condition characterized by sudden onset of paralysis and weakness in extremities (lower > upper). AFM affects the area of the spinal cord called gray matter, which causes the muscles and reflexes in the body to become weak. It is typically a lower motor neuron lesion but cases have been identified where there was reported involvement of the midbrain and medulla oblongata. The risk of getting AFM varies by age and year. There has been an increased number of AFM cases every two years since 2014 and mostly in young children. Still, CDC estimates that less than one to two in a million children in the United States will get AFM every year. AFM should be differentiated from other diseases which present with muscle weakness, hypotonia and flaccid paralysis. AFM has been known to be associated with viral infections such as West Nile virus, coxsackie virus, Adenovirus, Poliovirus, Enterovirus D71 and Enterovirus D68 as well as environmental toxins. The clinical course of the disease is characterized by a prodormal phase, progressive neurological injury phase and a convalescent phase. There is no specific treatment for AFM and prevention is aimed at measures which curtail the rate of infection by viruses known to be associated with AFM.
Historical Perspective
- In 2014, physicians in California and Colorado (USA) noted an increase in the number of patients presenting with the acute onset of flaccid paralysis and MRI findings consistent with lesions in the gray matter of the spinal cord.
- In 2014, a total of 120 cases were identified in the US and 22 were identified in 2015
- In 2016, 145 cases of AFM were diagnosed across the USA.
Causes
Acute flaccid myelitis (AFM) may be caused by viral infections or environmental toxins. The following viruses are known to be associated with AFM:[1][2][3][4][5]
- West Nile Virus
- Coxsackievirus (A24)
- Adenovirus
- Enterovirus 71 (EV 71) and Entervirus D68 (EV 68)
Differentiating Acute Flaccid Myelitis From Other Diseases
The following table differentiates acute flaccid myelitis from other diseases that cause muscle weakness, hypotonia, and flaccid paralysis:[6][6][7][8][1][9][10][11][12][13][14][15][16][17][18][19][20]
| Diseases | History and Physical | Diagnostic tests | Other Findings | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Motor Deficit | Sensory deficit | Cranial nerve Involvement | Autonomic dysfunction | Proximal/Distal/Generalized | Ascending/Descending/Systemic | Unilateral (UL)
or Bilateral (BL) or No Lateralization (NL) |
Onset | Lab or Imaging Findings | Specific test | ||
| Acute Flaccid Myelitis | + | + | + | - | Proximal > Distal | Ascending | UL/BL | Sudden | MRI (Longitudinal hyperintense lesions) | MRI and CSF PCR for viral etiology | Drooping eyelids
Difficulty swallowing Respiratory failure |
| Adult Botulism | + | - | + | + | Generalized | Descending | BL | Sudden | Toxin test | Blood, Wound, or Stool culture | Diplopia, Hyporeflexia, Hypotonia, possible respiratory paralysis |
| Infant Botulism | + | - | + | + | Generalized | Descending | BL | Sudden | Toxin test | Blood, Wound, or Stool culture | Flaccid paralysis (Floppy baby syndrome), possible respiratory paralysis |
| Guillian-Barre syndrome | + | - | - | - | Generalized | Ascending | BL | Insidious | CSF: ↑Protein
↓Cells |
Clinical & Lumbar Puncture | Progressive ascending paralysis following infection, possible respiratory paralysis |
| Eaton Lambert syndrome | + | - | + | + | Generalized | Systemic | BL | Intermittent | EMG, repetitive nerve stimulation test (RNS) | Voltage gated calcium channel (VGCC) antibody | Diplopia, ptosis, improves with movement (as the day progresses) |
| Myasthenia gravis | + | - | + | + | Generalized | Systemic | BL | Intermittent | EMG, Edrophonium test | Ach receptor antibody | Diplopia, ptosis, worsening with movement (as the day progresses) |
| Electrolyte disturbance | + | + | - | - | Generalized | Systemic | BL | Insidious | Electrolyte panel | ↓Ca++, ↓Mg++, ↓K+ | Possible arrhythmia |
| Organophosphate toxicity | + | + | - | + | Generalized | Ascending | BL | Sudden | Clinical diagnosis: physical exam & history | Clinical suspicion confirmed with RBC AchE activity | History of exposure to insecticide or living in farming environment. with : Diarrhea, Urination, Miosis, Bradycardia, Lacrimation, Emesis, Salivation, Sweating |
| Tick paralysis (Dermacentor tick) | + | - | - | - | Generalized | Ascending | BL | Insidious | Clinical diagnosis: physical exam & history | - | History of outdoor activity in Northeastern United States. The tick is often still latched to the patient at presentation (often in head and neck area) |
| Tetrodotoxin poisoning | + | - | + | + | Generalized | Systemic | BL | Sudden | Clinical diagnosis: physical exam & dietary history | - | History of consumption of puffer fish species. |
| Stroke | +/- | +/- | +/- | +/- | Generalized | Systemic | UL | Sudden | MRI +ve for ischemia or hemorrhage | MRI | Sudden unilateral motor and sensory deficit in a patient with a history of atherosclerotic risk factors (diabetes, hypertension, smoking) or atrial fibrillation. |
| Poliomyelitis | + | + | + | +/- | Proximal > Distal | Systemic | BL or UL | Sudden | PCR of CSF | Asymmetric paralysis following a flu-like syndrome. | |
| Transverse myelitis | + | + | + | + | Proximal > Distal | Systemic | BL or UL | Sudden | MRI & Lumbar puncture | MRI | History of chronic viral or autoimmune disease (e.g. HIV) |
| Neurosyphilis | + | + | - | +/- | Generalized | Systemic | BL | Insidious | MRI & Lumbar puncture | CSF VDRL-specifc
CSF FTA-Ab -sensitive |
History of unprotected sex or multiple sexual partners.
History of genital ulcer (chancre), diffuse maculopapular rash. |
| Muscular dystrophy | + | - | - | - | Proximal > Distal | Systemic | BL | Insidious | Genetic testing | Muscle biopsy | Progressive proximal lower limb weakness with calf pseudohypertrophy in early childhood. Gower sign positive. |
| Multiple sclerosis exacerbation | + | + | + | + | Generalized | Systemic | NL | Sudden | ↑CSF IgG levels
(monoclonal) |
Clinical assessment and MRI | Blurry vision, urinary incontinence, fatigue |
| Amyotrophic lateral sclerosis | + | - | - | - | Generalized | Systemic | BL | Insidious | Normal LP (to rule out DDx) | MRI & LP | Patient initially presents with upper motor neuron deficit (spasticity) followed by lower motor neuron deficit (flaccidity). |
| Inflammatory myopathy | + | - | - | - | Proximal > Distal | Systemic | UL or BL | Insidious | Elevated CK & Aldolase | Muscle biopsy | Progressive proximal muscle weakness in 3rd to 5th decade of life. With or without skin manifestations. |
Epidemiology and Demographics
Incidence and Prevalence
USA
- From June 1, 2012 to July 31, 2015, 59 reported cases met the California Department of Public Health (CDPH) case definition of acute flaccid myelitis (AFM), which defines AFM as acute onset of flaccid limb paralysis along with a lesion in the gray matter of the spinal cord.[8]
- Between August and December of 2014, 120 children from 34 US states met the criteria of AFM as outlined by the CDC.[21]
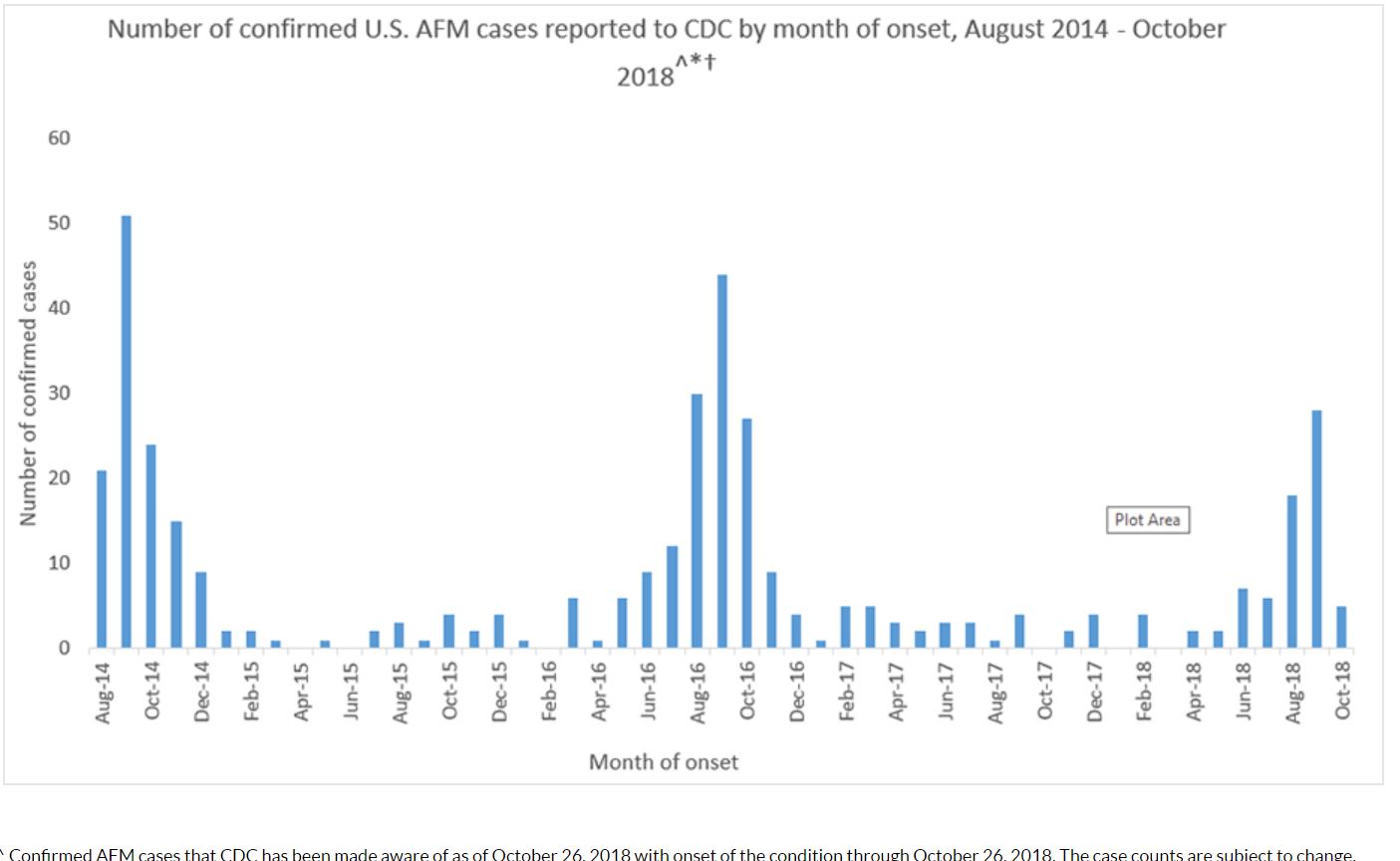
- From August 2014 through October 2018, CDC has received information on a total of 404 confirmed cases of acute flaccid myelitis (AFM) across the US; most of the cases have occurred in children[22]
- In 2015, 22 people were confirmed to have AFM. (Note: The cases occurred in 17 states across the U.S.)
- In 2016, 149 people were confirmed to have AFM. (Note: The cases occurred in 39 states across the U.S. and DC)
- In 2017, CDC received information for 33 confirmed cases of AFM. (Note: The cases occurred in 16 states across the U.S.)
- In October 2018, the New York State Department of Health (NYSDOH) confirmed 39 cases of the enterovirus EV-D68 in children across the state which was associated with an increase in number of cases of AFM.[23]
Age
- AFM affects older children. Median age at presentation is 9 years.[24]
Gender
- Male children are affected more frequently than females.
Risk Factors
The following risk factors increase the risk of development of AFM:
- Pulmonary comorbities (Asthma, COPD)
- Immunocompromised states (Immunosuppressants, organ transplantation, diabetes, systemic autoimmune diseases, hematologic malignancies)
Natural History, Complications and Prognosis
Natural History
- The clinical course of AFM typically consists of three phases:
- Prodormal phase
- Progressive neurological injury phase
- Convalescent phase
Prodormal phase
- A prodromal, often febrile, illness often precedes the onset of neurological deficits in most patients by a median of 5 days.
- The prodormal period consists of respiratory symptoms such as rhinorrhea, cough, or pharyngitis and/or gastrotestinal symptoms such as nausea, vomiting or diarrhea.
- Some patients report clinical improvement in their preceding illness before return of fever accompanied by headaches, stiff neck, or pain in the neck, back, or affected limb around the time of neurological deficit onset
Progressive neurological injury
- The pattern of limb weakness in AFM corresponds to a lower motor neuron lesion and upper extremities are more commonly affected.
- Involvement of cranial nerves points towards lesions in the cranial nerve motor nuclei of the brainstem
- On CSF examination, mild pleocytosis with mildly elevated protein may be seen
- On MRI, confluent, longitudinally extensive gray matter lesions may be seen in spinal cord. This may progress to nerve root enhancement on MRI in 2-3 weeks.
- On electromyography (EMG), reduced recruitment of motor unit potentials (MUPs) and low amplitude of compound muscle action potentials may be seen.
Convalescent phase
- Months after the initial acute neurological illness, patients suffering from AFM may smild improvements with rehabilitation therapies.
- On CSF examination, pleocytosis may resolve and protein remains elevated
- On EMG, fibrillations may be seen
Complications
AFM may lead to the development of following complications:
- Respiratory failure
- Urinary tract infections
- Skin ulcers and traumatic injury (specially in the affected extremity)
- Progressive muscular atrophy
Prognosis
- Physical and occupational therapy are especially important during recovery from AFM
- The extent of recovery varies. Although some people may make a full recovery, most have continued muscle weakness even after a year.
- Long term outcomes for patients suffering from AFM are not known.
Diagnosis
Diagnostic Criteria
The following table describes the case definition of AFM as outlined by the CDC:[25]
| Diagnosis | Criteria |
|---|---|
| Confirmed |
AND
|
| Probable |
AND
|
Symptoms
The following are the symptoms of acute flaccid myelitis:[26][27][1]
- Acute onset of flaccid limb paralysis (asymmetric)
- Fever
- Pain in the paralytic limb
- Eyelid drooping
- Difficulty with swallowing or slurred speech
- Cranial nerve abnormalities
- Headache
- Neck pain
- Bowel/bladder changes
Physical Examination
Temperature
- A fever is often present
Extremities
- Weakness of the extremities, predominantly of the proximal muscles, is characteristic of this form of the disease. Lower extremities are more often involved.
- Asymmetrical flaccid paralysis, predominantly of the proximal muscles, is characteristic of this form of the disease. Lower extremities are more often involved.
Neurologic
- Meningeal signs may be present on physical exam, such as:
- Initially hyperactive deep tendon reflexes, that later become absent.
- Common combinations of limb involvement include:
- One lower limb, followed by one upper limb
- Both lower limbs, followed by both upper limbs
- Quadriplegia is a rare finding in infants.
Laboratory Findings
Non specific tests
Blood tests
It is performed routinely to any suspected meningitis patients. It includes the following:
Virus detection
Virus may be detected through two ways:
- Samples can be taken by different ways from the suspected meningitis patients to detect the virus causing the disease.They may be collected for testing by:
- Polymerase Chain Reaction (PCR) can be used to detect the viruses in the blood. It can detect the DNA of the viruses like the enteroviruses and herpes simplex viruses.
Specific tests
Specific diagnostic tests include lumbar puncture with CSF examination. CSF examination findings in viral meningitis are as follows:
| Cerebrospinal fluid level | Normal level | Viral meningitis |
|---|---|---|
| Cells/ul | < 5 | >100 |
| Cells | Lymphos:Monos 7:3 | Lymphocytes>granulocytes |
| Total protein (mg/dl) | 45-60 | Normal or slightly elevated |
| Glucose ratio (CSF/plasma) | > 0.5 | >0.6 |
| Lactate (mmols/l) | < 2.1 | < 2.1 |
| Others | ICP:6-12 (cm H2O) | Throat swap |
Video explaining the lumbar puncture procedure: {{#ev:youtube|weoY_9tOcJQ}}
Imaging Findings
Magnetic resonance imaging (MRI) may reveal the following findings in patients suffering from AFM:
- Longitudinal hyperintense gray matter lesions in the spinal cord (may span more than 1 spinal segments).
- Hyperintense brainstem lesions ranging from midbrain to medulla oblongata.
Treatment
Medical Therapy
- There is no specific treatment for AFM.
- Treatments that have been tried include:
Corticosteroids
- There is no indication that corticosteroids should be either preferred or avoided in the treatment of AFM.
- There is no clear human evidence for efficacy of steroids in the treatment of AFM, and there is some evidence in a mouse model with EV-D68 that steroids may be harmful.
- The possible benefits of the use of corticosteroids to manage spinal cord edema or white matter involvement in AFM should be balanced with the possible harm due to immunosuppression in the setting of possible viral infection.
Intravenous immunoglobulin (IVIG)
- There is no indication that IVIG should be either preferred or avoided in the treatment of AFM.
- There is no clear human evidence for efficacy of IVIG in the treatment of AFM; evidence for efficacy is based on early treatment in animal models and it has not been given in a systematic manner to AFM patients to allow for measurements of efficacy.
- There is no evidence that treatment with IVIG is likely to be harmful.
Plasmapheresis
- There is no indication that plasma exchange should be either preferred or avoided in the treatment of AFM.
- There is no clear human evidence for efficacy of plasma exchange in the treatment of AFM, and it has not been given in a systematic manner to AFM patients to allow for measurements of efficacy.
- Although there are inherent procedure-associated risks, there is no evidence that using plasma exchange for patients with AFM is likely to be harmful.
Fluoxetine
- There is no indication that fluoxetine should be used for the treatment of AFM.
- There is no clear human evidence for efficacy of fluoxetine in the treatment of AFM based on a single retrospective evaluation conducted in patients with AFM, and data from a mouse model also did not support efficacy.
Antiviral medications
- There is no indication that antivirals should be used for the treatment of AFM, unless there is suspicion of herpesvirus infection (e.g., concomitant supra-tentorial disease or other clinical or radiologic features of herpesvirus infection).
- Appropriate antiviral medications (i.e., acyclovir, ganciclovir) should be empirically administered until herpesvirus infection has been excluded.
Interferon
- There is no indication that interferon should be used for the treatment of AFM, and there is concern about the potential for harm from the use of interferon given the immunomodulatory effects in the setting of possible ongoing viral replication.
Other immunosuppressive medications/biological modifiers
- There is no indication that biologic modifiers and the use of other immunosuppressive agents should be used for the treatment of AFM, and there is a possibility of harm in their use
Prevention
- There is no specific action to take to prevent AFM.
- Certain viruses are known to cause AFM including enteroviruses, such as poliovirus and enterovirus A71 (EV-A71), and West Nile virus.
- Poliovirus infection can be prevented by getting vaccinated. Polio vaccine contains inactivated (not live) virus, and protects against poliovirus. This vaccine does not protect against other viruses that may cause AFM.
- Protection from mosquito bites, which can carry West Nile virus, can be achieved by using mosquito repellent, staying indoors at dusk and dawn (when bites are more common), and removing standing or stagnant water (where mosquitoes can breed).
- Protection from enteroviruses by washing hands often with soap and water, avoiding close contact with people who are sick, and cleaning and disinfecting frequently touched surfaces, including toys.
References
- ↑ 1.0 1.1 1.2 Chong PF, Kira R, Mori H, Okumura A, Torisu H, Yasumoto S, Shimizu H, Fujimoto T, Hanaoka N, Kusunoki S, Takahashi T, Oishi K, Tanaka-Taya K (February 2018). "Clinical Features of Acute Flaccid Myelitis Temporally Associated With an Enterovirus D68 Outbreak: Results of a Nationwide Survey of Acute Flaccid Paralysis in Japan, August-December 2015". Clin. Infect. Dis. 66 (5): 653–664. doi:10.1093/cid/cix860. PMC 5850449. PMID 29028962.
- ↑ Morrey JD, Wang H, Hurst BL, Zukor K, Siddharthan V, Van Wettere AJ, Sinex DG, Tarbet EB (January 2018). "Causation of Acute Flaccid Paralysis by Myelitis and Myositis in Enterovirus-D68 Infected Mice Deficient in Interferon αβ/γ Receptor Deficient Mice". Viruses. 10 (1). doi:10.3390/v10010033. PMC 5795446. PMID 29329211.
- ↑ Maramattom BV, Philips G, Sudheesh N, Arunkumar G (January 2014). "Acute flaccid paralysis due to West nile virus infection in adults: A paradigm shift entity". Ann Indian Acad Neurol. 17 (1): 85–8. doi:10.4103/0972-2327.128561. PMC 3992778. PMID 24753667.
- ↑ "Coxsackie virus A24 infection presenting as acute flaccid paralysis - The Lancet" (PDF).
- ↑ Ooi MH, Wong SC, Clear D, Perera D, Krishnan S, Preston T, Tio PH, Willison HJ, Tedman B, Kneen R, Cardosa MJ, Solomon T (March 2003). "Adenovirus type 21-associated acute flaccid paralysis during an outbreak of hand-foot-and-mouth disease in Sarawak, Malaysia". Clin. Infect. Dis. 36 (5): 550–9. doi:10.1086/367648. PMID 12594634.
- ↑ 6.0 6.1 Kira R (February 2018). "[Acute Flaccid Myelitis]". Brain Nerve (in Japanese). 70 (2): 99–112. doi:10.11477/mf.1416200962. PMID 29433111.
- ↑ Hopkins SE (November 2017). "Acute Flaccid Myelitis: Etiologic Challenges, Diagnostic and Management Considerations". Curr Treat Options Neurol. 19 (12): 48. doi:10.1007/s11940-017-0480-3. PMID 29181601.
- ↑ 8.0 8.1 Messacar K, Schreiner TL, Van Haren K, Yang M, Glaser CA, Tyler KL, Dominguez SR (September 2016). "Acute flaccid myelitis: A clinical review of US cases 2012-2015". Ann. Neurol. 80 (3): 326–38. doi:10.1002/ana.24730. PMC 5098271. PMID 27422805.
- ↑ Messacar K, Asturias EJ, Hixon AM, Van Leer-Buter C, Niesters H, Tyler KL, Abzug MJ, Dominguez SR (August 2018). "Enterovirus D68 and acute flaccid myelitis-evaluating the evidence for causality". Lancet Infect Dis. 18 (8): e239–e247. doi:10.1016/S1473-3099(18)30094-X. PMID 29482893. Vancouver style error: initials (help)
- ↑ Chen IJ, Hu SC, Hung KL, Lo CW (September 2018). "Acute flaccid myelitis associated with enterovirus D68 infection: A case report". Medicine (Baltimore). 97 (36): e11831. doi:10.1097/MD.0000000000011831. PMC 6133480. PMID 30200066.
- ↑ "Botulism | Botulism | CDC".
- ↑ McCroskey LM, Hatheway CL (May 1988). "Laboratory findings in four cases of adult botulism suggest colonization of the intestinal tract". J. Clin. Microbiol. 26 (5): 1052–4. PMC 266519. PMID 3290234.
- ↑ Lindström M, Korkeala H (April 2006). "Laboratory diagnostics of botulism". Clin. Microbiol. Rev. 19 (2): 298–314. doi:10.1128/CMR.19.2.298-314.2006. PMC 1471988. PMID 16614251.
- ↑ Brook I (2006). "Botulism: the challenge of diagnosis and treatment". Rev Neurol Dis. 3 (4): 182–9. PMID 17224901.
- ↑ Dimachkie MM, Barohn RJ (May 2013). "Guillain-Barré syndrome and variants". Neurol Clin. 31 (2): 491–510. doi:10.1016/j.ncl.2013.01.005. PMC 3939842. PMID 23642721.
- ↑ Walling AD, Dickson G (February 2013). "Guillain-Barré syndrome". Am Fam Physician. 87 (3): 191–7. PMID 23418763.
- ↑ Gilhus NE (2011). "Lambert-eaton myasthenic syndrome; pathogenesis, diagnosis, and therapy". Autoimmune Dis. 2011: 973808. doi:10.4061/2011/973808. PMC 3182560. PMID 21969911.
- ↑ Krishnan C, Kaplin AI, Deshpande DM, Pardo CA, Kerr DA (May 2004). "Transverse Myelitis: pathogenesis, diagnosis and treatment". Front. Biosci. 9: 1483–99. PMID 14977560.
- ↑ Amato AA, Greenberg SA (December 2013). "Inflammatory myopathies". Continuum (Minneap Minn). 19 (6 Muscle Disease): 1615–33. doi:10.1212/01.CON.0000440662.26427.bd. PMID 24305450.
- ↑ Berger JR, Dean D (2014). "Neurosyphilis". Handb Clin Neurol. 121: 1461–72. doi:10.1016/B978-0-7020-4088-7.00098-5. PMID 24365430.
- ↑ "Acute Flaccid Myelitis | AFM Surveillance | CDC".
- ↑ McKay SL, Lee AD, Lopez AS, Nix WA, Dooling KL, Keaton AA, Spence-Davizon E, Herlihy R, Clark TA, Hopkins SE, Pastula DM, Sejvar J, Oberste MS, Pallansch MA, Patel M, Routh JA (November 2018). "Increase in Acute Flaccid Myelitis - United States, 2018". MMWR Morb. Mortal. Wkly. Rep. 67 (45): 1273–1275. doi:10.15585/mmwr.mm6745e1. PMID 30439867.
- ↑ "NYS Department of Health Confirms Cases of Serious Respiratory Virus".
- ↑ Nelson GR, Bonkowsky JL, Doll E, Green M, Hedlund GL, Moore KR, Bale JF (February 2016). "Recognition and Management of Acute Flaccid Myelitis in Children". Pediatr. Neurol. 55: 17–21. doi:10.1016/j.pediatrneurol.2015.10.007. PMID 26621554.
- ↑ "Acute Flaccid Myelitis | Case Definitions for Healthcare Professionals | CDC".
- ↑ "Acute Flaccid Myelitis in U.S. Children | Features | CDC".
- ↑ Andersen EW, Kornberg AJ, Freeman JL, Leventer RJ, Ryan MM (August 2017). "Acute flaccid myelitis in childhood: a retrospective cohort study". Eur. J. Neurol. 24 (8): 1077–1083. doi:10.1111/ene.13345. PMID 28639345.